Anticancer-active 3,4-diarylthiolated maleimides synthesis via three-component radical diarylthiolation
- PMID: 36505738
- PMCID: PMC9732252
- DOI: 10.3389/fchem.2022.1089860
Anticancer-active 3,4-diarylthiolated maleimides synthesis via three-component radical diarylthiolation
Abstract
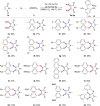
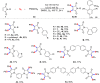
Herein, we report an efficient and simple copper-catalyzed oxidative diarylthiolation of maleimides with sulfur powder and aryl boronic acids, in which S powder was used as a substrate and internal oxidant. The corresponding double C-S bonds coupling products were obtained in moderate to high yields under a simple catalytic system. Mechanistic studies indicated that copper-catalyzed radical thiolation of aryl boronic acids with S powder, and the resulting arylthiyl underwent radical addition with double bonds of maleimides.
Keywords: bisthiolation; copper-catalyzed; maleimides; radical thiolation; sulfur powder.
Copyright © 2022 Wang, Li, Ma, Xia, Wang and Yu.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Oxidative Aminoarylselenation of Maleimides via Copper-Catalyzed Four-Component Cross-Coupling.Org Lett. 2019 Feb 1;21(3):745-748. doi: 10.1021/acs.orglett.8b03980. Epub 2019 Jan 14. Org Lett. 2019. PMID: 30638019
-
Copper(I)-Catalyzed Three-Component Selenosulfonation of Maleimides with Sulfonyl Hydrazides and Diselenides via Radical Relay.J Org Chem. 2022 Nov 18;87(22):15661-15669. doi: 10.1021/acs.joc.2c01907. Epub 2022 Nov 1. J Org Chem. 2022. PMID: 36317696
-
Copper-Catalyzed Oxidative Thioamination of Maleimides with Amines and Bunte Salts.Org Lett. 2020 Mar 6;22(5):1863-1867. doi: 10.1021/acs.orglett.0c00207. Epub 2020 Feb 7. Org Lett. 2020. PMID: 32031819
-
Cu-Catalyzed Diarylthiolation of Ynones with Aryl Iodides and Elemental Sulfur: An Access to Tetrasubstituted (Z)-1,2-Bis(arylthio)alkenes and Benzo[b][1,4]dithiines.J Org Chem. 2022 Sep 2;87(17):11796-11804. doi: 10.1021/acs.joc.2c01575. Epub 2022 Aug 22. J Org Chem. 2022. PMID: 35993485
-
Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source.Acc Chem Res. 2014 May 20;47(5):1513-22. doi: 10.1021/ar4003202. Epub 2014 Apr 28. Acc Chem Res. 2014. PMID: 24773518 Review.
Cited by
-
MasitinibL shows promise as a drug-like analog of masitinib that elicits comparable SARS-Cov-2 3CLpro inhibition with low kinase preference.Sci Rep. 2023 Apr 28;13(1):6972. doi: 10.1038/s41598-023-33024-2. Sci Rep. 2023. PMID: 37117213 Free PMC article.
References
-
- Ananikov V. P., Beletskaya I. P. (2004a). Palladium-catalyzed addition of disulfides and diselenides to alkynes under solvent free conditionsElectronic supplementary information (ESI) available: Full experimental details of synthetic procedure, compound separation and purification, details of spectroscopic studies, kinetic measurements and compound characterization. See http://www.rsc.org/suppdata/ob/b3/b312471a/. Org. Biomol. Chem. 2, 284–287. 10.1039/B312471A - DOI - PubMed
-
- Ananikov V. P., Gayduk K. A., Beletskaya I. P., Khrustalev V. N., Antipin M. Y. (2008b). Remarkable ligand effect in Ni- and Pd-Catalyzed bisthiolation and bisselenation of terminal alkynes solving the problem of stereoselective dialkyldichalcogenide addition to the C≡C bond. Chem. Eur. J. 14, 2420–2434. 10.1002/chem.200701278 - DOI - PubMed
-
- Cai M., Wang Y., Hao W. (2007e). Palladium-catalyzed addition of diaryl disulfides and diselenides to terminal alkynes in room temperature ionic liquids. Green Chem. 9, 1180–1184. 10.1039/B706320B - DOI
LinkOut - more resources
Full Text Sources

