Oncolytic virus: A catalyst for the treatment of gastric cancer
- PMID: 36505792
- PMCID: PMC9731121
- DOI: 10.3389/fonc.2022.1017692
Oncolytic virus: A catalyst for the treatment of gastric cancer
Abstract
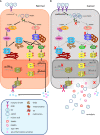
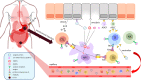
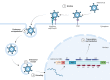
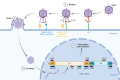
Gastric cancer (GC) is a leading contributor to global cancer incidence and mortality. According to the GLOBOCAN 2020 estimates of incidence and mortality for 36 cancers in 185 countries produced by the International Agency for Research on Cancer (IARC), GC ranks fifth and fourth, respectively, and seriously threatens the survival and health of people all over the world. Therefore, how to effectively treat GC has become an urgent problem for medical personnel and scientific workers at this stage. Due to the unobvious early symptoms and the influence of some adverse factors such as tumor heterogeneity and low immunogenicity, patients with advanced gastric cancer (AGC) cannot benefit significantly from treatments such as radical surgical resection, radiotherapy, chemotherapy, and targeted therapy. As an emerging cancer immunotherapy, oncolytic virotherapies (OVTs) can not only selectively lyse cancer cells, but also induce a systemic antitumor immune response. This unique ability to turn unresponsive 'cold' tumors into responsive 'hot' tumors gives them great potential in GC therapy. This review integrates most experimental studies and clinical trials of various oncolytic viruses (OVs) in the diagnosis and treatment of GC. It also exhaustively introduces the concrete mechanism of invading GC cells and the viral genome composition of adenovirus and herpes simplex virus type 1 (HSV-1). At the end of the article, some prospects are put forward to determine the developmental directions of OVTs for GC in the future.
Keywords: adenovirus; combination therapy; gastric cancer; herpes simplex virus type 1; oncolytic virus; tumor microenvironment.
Copyright © 2022 Wang, Du and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Gastric Cancer and Viruses: A Fine Line between Friend or Foe.Vaccines (Basel). 2022 Apr 13;10(4):600. doi: 10.3390/vaccines10040600. Vaccines (Basel). 2022. PMID: 35455349 Free PMC article. Review.
-
Novel Oncolytic Herpes Simplex Virus 1 VC2 Promotes Long-Lasting, Systemic Anti-melanoma Tumor Immune Responses and Increased Survival in an Immunocompetent B16F10-Derived Mouse Melanoma Model.J Virol. 2021 Jan 13;95(3):e01359-20. doi: 10.1128/JVI.01359-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177208 Free PMC article.
-
Herpes simplex virus 1 as an oncolytic viral therapy for refractory cancers.Front Oncol. 2022 Jul 27;12:940019. doi: 10.3389/fonc.2022.940019. eCollection 2022. Front Oncol. 2022. PMID: 35965554 Free PMC article. Review.
-
Oncolytic Viro-Immunotherapy: An Emerging Option in the Treatment of Gliomas.Front Immunol. 2021 Oct 5;12:721830. doi: 10.3389/fimmu.2021.721830. eCollection 2021. Front Immunol. 2021. PMID: 34675919 Free PMC article. Review.
-
Oncolytic Virus Therapy in a New Era of Immunotherapy, Enhanced by Combination with Existing Anticancer Therapies: Turn up the Heat!J Cancer. 2025 Feb 18;16(6):1782-1793. doi: 10.7150/jca.102285. eCollection 2025. J Cancer. 2025. PMID: 40092697 Free PMC article. Review.
Cited by
-
Immunotherapy in gastrointestinal cancers: current strategies and future directions - a literature review.Ann Med Surg (Lond). 2025 Jan 9;87(1):151-160. doi: 10.1097/MS9.0000000000002757. eCollection 2025 Jan. Ann Med Surg (Lond). 2025. PMID: 40109582 Free PMC article. Review.
-
HSV: The scout and assault for digestive system tumors.Front Mol Biosci. 2023 Feb 28;10:1142498. doi: 10.3389/fmolb.2023.1142498. eCollection 2023. Front Mol Biosci. 2023. PMID: 36926680 Free PMC article. Review.
-
Retroviral Replicating Vectors Mediated Prodrug Activator Gene Therapy in a Gastric Cancer Model.Int J Mol Sci. 2023 Oct 2;24(19):14823. doi: 10.3390/ijms241914823. Int J Mol Sci. 2023. PMID: 37834271 Free PMC article.
-
Research progress on mechanisms of tumor immune microenvironment and gastrointestinal resistance to immunotherapy: mini review.Front Immunol. 2025 Jul 25;16:1641518. doi: 10.3389/fimmu.2025.1641518. eCollection 2025. Front Immunol. 2025. PMID: 40787471 Free PMC article. Review.
-
Classical biomarkers and non-coding RNAs associated with diagnosis and treatment in gastric cancer.Oncol Res. 2025 Apr 18;33(5):1069-1089. doi: 10.32604/or.2025.063005. eCollection 2025. Oncol Res. 2025. PMID: 40296904 Free PMC article. Review.
References
-
- Kang YK, Chin K, Chung HC, Kadowaki S, Oh SC, Nakayama N, et al. . S-1 plus leucovorin and oxaliplatin versus s-1 plus cisplatin as first-line therapy in patients with advanced gastric cancer (Solar): A randomised, open-label, phase 3 trial. Lancet Oncol (2020) 21(8):1045–56. doi: 10.1016/s1470-2045(20)30315-6 - DOI - PubMed
-
- Wiedermann U, Garner-Spitzer E, Chao Y, Maglakelidze M, Bulat I, Dechaphunkul A, et al. . Clinical and immunologic responses to a b-cell epitope vaccine in patients with Her2/Neu-overexpressing advanced gastric cancer-results from phase ib trial Imu.Acs.001. Clin Cancer Res (2021) 27(13):3649–60. doi: 10.1158/1078-0432.Ccr-20-3742 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

