A novel prediction model for pathological complete response based on clinical and blood parameters in locally advanced rectal cancer
- PMID: 36505836
- PMCID: PMC9727231
- DOI: 10.3389/fonc.2022.932853
A novel prediction model for pathological complete response based on clinical and blood parameters in locally advanced rectal cancer
Abstract
Background: The aim of this study was to investigate whether clinical and blood parameters can be used for predicting pathological complete response (pCR) to neoadjuvant chemoradiotherapy (nCRT) in patients with locally advanced rectal cancer (LARC).
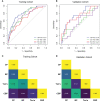
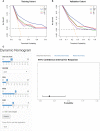
Methods: We retrospectively enrolled 226 patients with LARC [allocated in a 7:3 ratio to a training (n = 158) or validation (n = 68) cohort] who received nCRT before radical surgery. Backward stepwise logistic regression was performed to identify clinical and blood parameters associated with achieving pCR. Models based on clinical parameters (CP), blood parameters (BP), and clinical-blood parameters (CBP) were constructed for comparison with previously reported Tan's model. The performance of the four models was evaluated by receiver operating characteristic (ROC) curve analysis, calibration, and decision curve analysis (DCA) in both cohorts. A dynamic nomogram was constructed for the presentation of the best model.
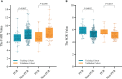
Results: The CP and BP models based on multivariate logistic regression analysis showed that interval, Grade, CEA and fibrinogen-albumin ratio index (FARI), sodium-to-globulin ratio (SGR) were the independent clinical and blood predictors for achieving pCR, respectively. The area under the ROC curve of the CBP model achieved a score of 0.818 and 0.752 in both cohorts, better than CP (0.762 and 0.589), BP (0.695 and 0.718), Tan (0.738 and 0.552). CBP also showed better calibration and DCA than other models in both cohorts. Moreover, CBP revealed significant improvement compared with other models in training cohort (P < 0.05), and CBP showed significant improvement compared with CP and Tan's model in validation cohort (P < 0.05).
Conclusion: We demonstrated that CBP predicting model have potential in predicting pCR to nCRT in patient with LARC.
Keywords: locally advanced rectal cancer; neoadjuvant chemotherapy; nomogram; pathological complete response; prediction model.
Copyright © 2022 Lu, Liu, Wang, Meng, Peng, Qu, Zhang, Fu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Preoperative Fibrinogen-Albumin Ratio Index (FARI) is a Reliable Prognosis and Chemoradiotherapy Sensitivity Predictor in Locally Advanced Rectal Cancer Patients Undergoing Radical Surgery Following Neoadjuvant Chemoradiotherapy.Cancer Manag Res. 2020 Sep 17;12:8555-8568. doi: 10.2147/CMAR.S273065. eCollection 2020. Cancer Manag Res. 2020. PMID: 32982448 Free PMC article.
-
Pretreatment blood biomarkers combined with magnetic resonance imaging predict responses to neoadjuvant chemoradiotherapy in locally advanced rectal cancer.Front Oncol. 2022 Aug 9;12:916840. doi: 10.3389/fonc.2022.916840. eCollection 2022. Front Oncol. 2022. PMID: 36016621 Free PMC article.
-
Prognostic value of mesorectal package area in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy: A retrospective cohort study.Front Oncol. 2022 Oct 3;12:941786. doi: 10.3389/fonc.2022.941786. eCollection 2022. Front Oncol. 2022. PMID: 36263216 Free PMC article.
-
Development and Validation of an MRI-Based Nomogram Model for Predicting Disease-Free Survival in Locally Advanced Rectal Cancer Treated With Neoadjuvant Radiotherapy.Front Oncol. 2021 Nov 15;11:784156. doi: 10.3389/fonc.2021.784156. eCollection 2021. Front Oncol. 2021. PMID: 34869040 Free PMC article.
-
Body composition parameters combined with blood biomarkers and magnetic resonance imaging predict responses to neoadjuvant chemoradiotherapy in locally advanced rectal cancer.Front Oncol. 2023 Nov 22;13:1242193. doi: 10.3389/fonc.2023.1242193. eCollection 2023. Front Oncol. 2023. PMID: 38074647 Free PMC article.
Cited by
-
Clinical Prediction Models for Contact X-Ray Brachytherapy in Managing Rectal Cancers: A Scoping Review.Cancer Med. 2025 Apr;14(7):e70697. doi: 10.1002/cam4.70697. Cancer Med. 2025. PMID: 40178039 Free PMC article.
-
Multiparametric magnetic resonance imaging (MRI)-based radiomics model explained by the Shapley Additive exPlanations (SHAP) method for predicting complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicenter retrospective study.Quant Imaging Med Surg. 2024 Jul 1;14(7):4617-4634. doi: 10.21037/qims-24-7. Epub 2024 Jun 11. Quant Imaging Med Surg. 2024. PMID: 39022292 Free PMC article.
-
Exploring novel genetic and hematological predictors of response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer.Front Genet. 2023 Aug 31;14:1245594. doi: 10.3389/fgene.2023.1245594. eCollection 2023. Front Genet. 2023. PMID: 37719698 Free PMC article.
-
Progress and current trends in prediction models for the occurrence and prognosis of cancer and cancer-related complications: a bibliometric and visualization analysis.Front Oncol. 2025 Jul 8;15:1556521. doi: 10.3389/fonc.2025.1556521. eCollection 2025. Front Oncol. 2025. PMID: 40697367 Free PMC article.
References
-
- Hoendervangers S, Burbach JPM, Lacle MM, Koopman M, van Grevenstein WMU, Intven MPW, et al. . Pathological complete response following different neoadjuvant treatment strategies for locally advanced rectal cancer: A systematic review and meta-analysis. Ann Surg Oncol (2020) 27(11):4319–36. doi: 10.1245/s10434-020-08615-2 - DOI - PMC - PubMed
-
- Polanco PM, Mokdad AA, Zhu H, Choti MA, Huerta S. Association of adjuvant chemotherapy with overall survival in patients with rectal cancer and pathologic complete response following neoadjuvant chemotherapy and resection. JAMA Oncol (2018) 4(7):938–43. doi: 10.1001/jamaoncol.2018.0231 - DOI - PMC - PubMed
-
- van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, et al. . Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the international watch & wait database (IWWD): an international multicentre registry study. Lancet (2018) 391(10139):2537–45. doi: 10.1016/S0140-6736(18)31078-X - DOI - PubMed
-
- Dattani M, Heald RJ, Goussous G, Broadhurst J, Sao Juliao GP, Habr-Gama A, et al. . Oncological and survival outcomes in watch and wait patients with a clinical complete response after neoadjuvant chemoradiotherapy for rectal cancer: A systematic review and pooled analysis. Ann Surg (2018) 268(6):955–67. doi: 10.1097/SLA.0000000000002761 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

