In vivo efficacy assessment of the CDK4/6 inhibitor palbociclib and the PLK1 inhibitor volasertib in human chordoma xenografts
- PMID: 36505864
- PMCID: PMC9732546
- DOI: 10.3389/fonc.2022.960720
In vivo efficacy assessment of the CDK4/6 inhibitor palbociclib and the PLK1 inhibitor volasertib in human chordoma xenografts
Abstract
Background: Management of advanced chordomas remains delicate considering their insensitivity to chemotherapy. Homozygous deletion of the regulatory gene CDKN2A has been described as the most frequent genetic alteration in chordomas and may be considered as a potential theranostic marker. Here, we evaluated the tumor efficacy of the CDK4/6 inhibitor palbociclib, as well as the PLK1 inhibitor volasertib, in three chordoma patient-derived xenograft (PDX) models to validate and identify novel therapeutic approaches.
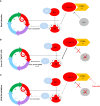
Methods: From our chordoma xenograft panel, we selected three models, two of them harboring a homozygous deletion of CDKN2A/2B genes, and the last one a PBRM1 pathogenic variant (as control). For each model, we tested the palbociclib and volasertib drugs with pharmacodynamic studies together with RT-PCR and RNAseq analyses.
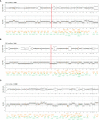
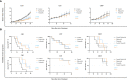
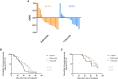
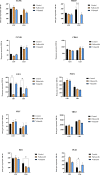
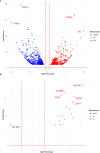
Results: For palbociclib, we observed a significant tumor response for one of two models harboring the deletion of CDKN2A/2B (p = 0.02), and no significant tumor response in the PBRM1-mutated PDX; for volasertib, we did not observe any response in the three tested models. RT-PCR and RNAseq analyses showed a correlation between cell cycle markers and responses to palbociclib; finally, RNAseq analyses showed a natural enrichment of the oxidative phosphorylation genes (OxPhos) in the palbociclib-resistant PDX (p = 0.02).
Conclusion: CDK4/6 inhibition appears as a promising strategy to manage advanced chordomas harboring a loss of CDKN2A/2B. However, further preclinical studies are strongly requested to confirm it and to understand acquired or de novo resistance to palbociclib, in the peculiar view of a targeting of the oxidative phosphorylation genes.
Keywords: CDK4/6 inhibitor; CDKN2A/2B deletion; PLK1 inhibitor; chordoma patient’s derived xenograft; palbocicib; volasertib.
Copyright © 2022 Passeri, Dahmani, Masliah-Planchon, El Botty, Courtois, Vacher, Marangoni, Nemati, Roman-Roman, Adle-Biassette, Mammar, Froelich, Bièche and Decaudin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

