Clinical features and therapeutic outcomes of alveolar soft part sarcoma in children: A single-center, retrospective study
- PMID: 36505873
- PMCID: PMC9730233
- DOI: 10.3389/fonc.2022.1019911
Clinical features and therapeutic outcomes of alveolar soft part sarcoma in children: A single-center, retrospective study
Abstract
Background: Alveolar soft part sarcoma (ASPS) is a rare sarcoma that has been shown to be highly effective to antiangiogenic agents and immune checkpoint inhibitors, but most reported studies about ASPS were concentrated on adult population. In this study, we aimed to describe the clinical features and therapeutic outcomes of ASPS in children.
Methods: We retrospectively reviewed the records of patients with ASPS in our institution since Jan 2015. All patients included in this study were pathologically confirmed ASPS and aged under 12 years at the time of initial diagnosis. Demographic characteristics, tumor sizes, primary tumor sites, metastasis, treatments used, therapeutic responses and survivals were evaluated.
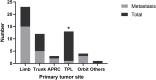
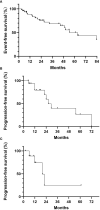
Results: We identified a total of 56 patients to be initially diagnosed as ASPS since Jan 2015. A predisposition of high occurrence in head and neck (32.1%) was observed (versus 41.1% in limbs and 21.4% in trunk). 26 (46.4%) patients developed metastasis at the time of diagnosis or during follow-up. Tumors in tongue, pharynx and larynx had the least likelihood to metastasize (7.7%, P<0.05). Observation was recommended for 15 stage IV patients with only pulmonary metastasis. 7 (46.7%) patients remained stable until last follow up. The 1-year PFS rate was 83.3% and median progression-free survival time (PFS) was 29.4 months. 15 patients with progressive disease received mono or combined therapy. 11 patients received PD-1 monotherapy. 2 patients achieved partial response and 5 stable disease. The overall response rate was 18.2%. The median PFS of this group was 22.0 months, and the 1-year PFS rate was 70.0%. 4 patients received a combination therapy of PD-1 inhibitors plus tyrosine kinase inhibitors. All of them remained stable. No disease-related death occurred during follow-up.
Conclusions: ASPS exhibits a higher occurrence in head and neck in children. ASPS originating from glossopharyngeal region tends to have a lower metastasis rate. ASPS displays a more indolent growth pattern in children, which makes observation a preferable choice for children with sole pulmonary metastasis. Pediatric ASPS appears to be less effective to targeted therapy and immunotherapy than adults. The treatment of progressive ASPS in children remains challenging.
Keywords: alveolar soft part sarcoma; clinical features; immunotherapy; pediatric; targeted therapy.
Copyright © 2022 Tan, Liu, Xue, Fan, Bai, Li, Gao, Zhang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer LX declared a shared parent affiliation with the authors to the handling editor at the time of review.
Figures
References
-
- Hodge JC, Pearce KE, Wang X, Wiktor AE, Oliveira AM, Greipp PT. Molecular cytogenetic analysis for TFE3 rearrangement in Xp11.2 renal cell carcinoma and alveolar soft part sarcoma: validation and clinical experience with 75 cases. Mod Pathol (2014) 27(1):113–27. - PubMed
LinkOut - more resources
Full Text Sources

