Stress-induced transcriptional readthrough into neighboring genes is linked to intron retention
- PMID: 36505935
- PMCID: PMC9732411
- DOI: 10.1016/j.isci.2022.105543
Stress-induced transcriptional readthrough into neighboring genes is linked to intron retention
Abstract
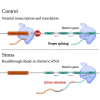
Exposure to certain stresses leads to readthrough transcription. Using polyA-selected RNA-seq in mouse fibroblasts subjected to heat shock, oxidative, or osmotic stress, we found that readthrough transcription can proceed into proximal downstream genes, in a phenomenon previously termed "read-in." We found that read-in genes share distinctive genomic characteristics; they are GC-rich and extremely short , with genomic features conserved in human. Using ribosome profiling, we found that read-in genes show significantly reduced translation. Strikingly, read-in genes demonstrate marked intron retention, mostly in their first introns, which could not be explained solely by their short introns and GC-richness, features often associated with intron retention. Finally, we revealed H3K36me3 enrichment upstream to read-in genes. Moreover, demarcation of exon-intron junctions by H3K36me3 was absent in read-in first introns. Our data portray a relationship between read-in and intron retention, suggesting they may have co-evolved to facilitate reduced translation of read-in genes during stress.
Keywords: Biological sciences; Molecular Genetics; Molecular biology; Molecular interaction.
© 2022 The Authors.
Conflict of interest statement
The authors declare no conflict of Interest.
Figures







References
-
- López-Maury L., Marguerat S., Bähler J. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat. Rev. Genet. 2008;9:583–593. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

