Efficacy and Safety of Inhalation of Nebulized Ethanol in COVID-19 Treatment: A Randomized Clinical Trial
- PMID: 36505954
- PMCID: PMC9728981
- DOI: 10.7759/cureus.32218
Efficacy and Safety of Inhalation of Nebulized Ethanol in COVID-19 Treatment: A Randomized Clinical Trial
Abstract
Background: Coronavirus disease 2019 (COVID-19) is a pandemic caused by the SARS-CoV-2 virus. Many efforts have been made and are currently being made to prevent and treat this global disease.
Objectives: This study was designed to evaluate the efficacy and safety of nebulized ethanol (EtOH) in treating COVID-19.
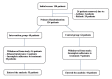
Methods: A randomized clinical trial (RCT) of 99 symptomatic and real-time polymerase chain reaction (RT-PCR)-positive patients admitted to a hospital receiving remdesivir-dexamethasone was conducted. They were randomly assigned to receive distilled water spray (control group (CG)) or 35% EtOH spray (intervention group (IG)). Both groups inhaled three puffs of spray (nebulizer) every six hours for a week. The primary outcome included Global Symptomatic Score (GSS) between the two groups at the first visit and on days three, seven, and 14. Secondary outcomes included the Clinical Status Scale (CSS; a seven-point ordinal scale ranging from death to complete recovery) and readmission rate.
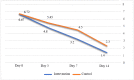
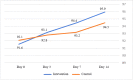
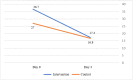
Results: A total of 44 and 55 patients were enrolled in the IG and CG, respectively. Although there was no difference at admission, the GSS and CSS improved significantly in the IG (p = 0.016 and p = 0.001, respectively). The IG readmission rate was considerably lower (0% vs. 10.9%; p = 0.02).
Conclusions: Inhaled-nebulized EtOH is effective in rapidly improving the clinical status and reducing further treatment. Due to its low cost, availability, and absent/tolerable adverse events, it could be recommended as an adjunctive treatment for moderate COVID-19. Further research on curative effects in more serious cases and in prevention is advisable.
Keywords: coronavirus disease; coronavirus disease 2019; covid-19; ethanol; inhalation; nebulized ethanol; nebulizer; sars-cov-2.
Copyright © 2022, Amoushahi et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Nebulised surfactant for the treatment of severe COVID-19 in adults (COV-Surf): A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Dec 10;21(1):1014. doi: 10.1186/s13063-020-04944-5. Trials. 2020. PMID: 33302976 Free PMC article. Clinical Trial.
-
Evaluating the efficacy and safety of human anti-SARS-CoV-2 convalescent plasma in severely ill adults with COVID-19: A structured summary of a study protocol for a randomized controlled trial.Trials. 2020 Jun 8;21(1):499. doi: 10.1186/s13063-020-04422-y. Trials. 2020. PMID: 32513308 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
Cited by
-
Efficacy and Safety of Inhaled Ethanol in Early-Stage SARS-CoV-2 Infection in Older Adults: A Phase II Randomized Clinical Trial.Pharmaceutics. 2023 Feb 16;15(2):667. doi: 10.3390/pharmaceutics15020667. Pharmaceutics. 2023. PMID: 36839987 Free PMC article.
-
Positive Correlation Between Heavy Alcoholic Drinking and SARS-Cov-2 Non-Infection Rate.Cureus. 2023 Jun 8;15(6):e40130. doi: 10.7759/cureus.40130. eCollection 2023 Jun. Cureus. 2023. PMID: 37304380 Free PMC article.
-
Ethanol Inhalation for Respiratory Infections due to Enveloped Viruses.Infect Dis Ther. 2025 Jun;14(6):1143-1156. doi: 10.1007/s40121-025-01157-8. Epub 2025 Apr 17. Infect Dis Ther. 2025. PMID: 40246793 Free PMC article. Review.
References
-
- Alcohol-based hand sanitisers as first line of defence against SARS-CoV-2: a review of biology, chemistry and formulations. Singh D, Joshi K, Samuel A, Patra J, Mahindroo N. https://doi.org/10.1017/S0950268820002319 Epidemiol Infect. 2020;148:0. - PMC - PubMed
-
- Antiviral activity of alcohol for surface disinfection. Moorer WR. Int J Dent Hyg. 2003;1:138–142. - PubMed
-
- Ethanol decreases inflammatory response in human lung epithelial cells by inhibiting the canonical NF-kB-pathway. Mörs K, Hörauf JA, Kany S, et al. https://doi.org/10.1159/000480313. Cell Physiol Biochem. 2017;43:17–30. - PubMed
-
- Acute ethanol administration results in a protective cytokine and neuroinflammatory profile in traumatic brain injury. Chandrasekar A, Heuvel FO, Palmer A, et al. https://doi.org/10.1016/j.intimp.2017.08.002. Int Immunopharmacol. 2017;51:66–75. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
