Human stem cell derived beta-like cells engineered to present PD-L1 improve transplant survival in NOD mice carrying human HLA class I
- PMID: 36506044
- PMCID: PMC9732725
- DOI: 10.3389/fendo.2022.989815
Human stem cell derived beta-like cells engineered to present PD-L1 improve transplant survival in NOD mice carrying human HLA class I
Abstract
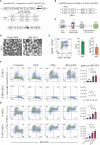
There is a critical need for therapeutic approaches that combine renewable sources of replacement beta cells with localized immunomodulation to counter recurrence of autoimmunity in type 1 diabetes (T1D). However, there are few examples of animal models to study such approaches that incorporate spontaneous autoimmunity directed against human beta cells rather than allogenic rejection. Here, we address this critical limitation by demonstrating rejection and survival of transplanted human stem cell-derived beta-like cells clusters (sBCs) in a fully immune competent mouse model with matching human HLA class I and spontaneous diabetes development. We engineered localized immune tolerance toward transplanted sBCs via inducible cell surface overexpression of PD-L1 (iP-sBCs) with and without deletion of all HLA class I surface molecules via beta-2 microglobulin knockout (iP-BKO sBCs). NOD.HLA-A2.1 mice, which lack classical murine MHC I and instead express human HLA-A*02:01, underwent transplantation of 1,000 human HLA-A*02:01 sBCs under the kidney capsule and were separated into HLA-A2 positive iP-sBC and HLA-class I negative iP-BKO sBC groups, each with +/- doxycycline (DOX) induced PD-L1 expression. IVIS imaging showed significantly improved graft survival in mice transplanted with PD-L1 expressing iP-sBC at day 3 post transplantation compared to controls. However, luciferase signal dropped below in vivo detection limits by day 14 for all groups in this aggressive immune competent diabetes model. Nonetheless, histological examination revealed significant numbers of surviving insulin+/PD-L1+ sBCs cells for DOX-treated mice at day 16 post-transplant despite extensive infiltration with high numbers of CD3+ and CD45+ immune cells. These results show that T cells rapidly infiltrate and attack sBC grafts in this model but that significant numbers of PD-L1 expressing sBCs manage to survive in this harsh immunological environment. This investigation represents one of the first in vivo studies recapitulating key aspects of human autoimmune diabetes to test immune tolerance approaches with renewable sources of beta cells.
Keywords: HLA Class I; PD-L1; beta cell replacement; humanized mouse; stem cell-derived beta cell; tolerance; type 1 diabetes.
Copyright © 2022 Santini-González, Castro-Gutierrez, Becker, Rancourt, Russ and Phelps.
Conflict of interest statement
HR is or has been a consultant and islets SAB member to Sigilon therapeutics, a SAB member at Prellis Biologics and consultant to Eli Lilly and Minutia. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Martin-Pagola A, Sisino G, Allende G, Dominguez-Bendala J, Gianani R, Reijonen H, et al. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia (2008) 51(10):1803–13. doi: 10.1007/s00125-008-1105-x - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

