Wnt signaling pathway inhibitors, sclerostin and DKK-1, correlate with pain and bone pathology in patients with Gaucher disease
- PMID: 36506070
- PMCID: PMC9730525
- DOI: 10.3389/fendo.2022.1029130
Wnt signaling pathway inhibitors, sclerostin and DKK-1, correlate with pain and bone pathology in patients with Gaucher disease
Abstract
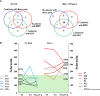
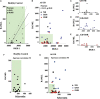
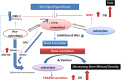
Patients with Gaucher disease (GD) have progressive bone involvement that clinically presents with debilitating bone pain, structural bone changes, bone marrow infiltration (BMI), Erlenmeyer (EM) flask deformity, and osteoporosis. Pain is referred by the majority of GD patients and continues to persist despite the type of therapy. The pain in GD is described as chronic deep penetrating pain; however, sometimes, patients experience severe acute pain. The source of bone pain is mainly debated as nociceptive pain secondary to bone pathology or neuropathic or inflammatory origins. Osteocytes constitute a significant source of secreted molecules that coordinate bone remodeling. Osteocyte markers, sclerostin (SOST) and Dickkopf-1 (DKK-1), inactivate the canonical Wnt signaling pathway and lead to the inhibition of bone formation. Thus, circulated sclerostin and DKK-1 are potential biomarkers of skeletal abnormalities. This study aimed to assess the circulating levels of sclerostin and DKK-1 in patients with GD and their correlation with clinical bone pathology parameters: pain, bone mineral density (BMD), and EM deformity. Thirty-nine patients with GD were classified into cohorts based on the presence and severity of bone manifestations. The serum levels of sclerostin and DKK-1 were quantified by enzyme-linked immunosorbent assays. The highest level of sclerostin was measured in GD patients with pain, BMI, and EM deformity. The multiparameter analysis demonstrated that 95% of GD patients with pain, BMI, and EM deformity had increased levels of sclerostin. The majority of patients with elevated sclerostin also have osteopenia or osteoporosis. Moreover, circulating sclerostin level increase with age, and GD patients have elevated sclerostin levels when compared with healthy control from the same age group. Pearson's linear correlation analysis showed a positive correlation between serum DKK-1 and sclerostin in healthy controls and GD patients with normal bone mineral density. However, the balance between sclerostin and DKK-1 waned in GD patients with osteopenia or osteoporosis. In conclusion, the osteocyte marker, sclerostin, when elevated, is associated with bone pain, BMI, and EM flask deformity in GD patients. The altered sclerostin/DKK-1 ratio correlates with the reduction of bone mineral density. These data confirm that the Wnt signaling pathway plays a role in GD-associated bone disease. Sclerostin and bone pain could be used as biomarkers to assess patients with a high risk of BMI and EM flask deformities.
Trial registration: ClinicalTrials.gov NCT04055831.
Keywords: DKK-1; Gaucher disease; SOST; Wnt; bone; osteoporosis; pain.
Copyright © 2022 Ivanova, Dao, Kasaci, Friedman, Noll and Goker-Alpan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Oliveri B, Gonzalez DC, Rozenfeld P, Ferrari E, Gutierrez G. Grupo de estudio bone involvement gaucher d. early diagnosis of gaucher disease based on bone symptoms. Medicina (B Aires). (2020) 80(5):487–94. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

