Effect of different timing of letrozole initiation on pregnancy outcome in polycystic ovary syndrome
- PMID: 36506073
- PMCID: PMC9731802
- DOI: 10.3389/fendo.2022.1059609
Effect of different timing of letrozole initiation on pregnancy outcome in polycystic ovary syndrome
Abstract
Objective: To investigate the efficacy of oral letrozole (LE) starting on day 3 or 5 of the menstrual cycle in patients with polycystic ovary syndrome (PCOS).
Design: Retrospective cohort study.
Setting: Reproductive Endocrinology Department of Hangzhou Women's Hospital.
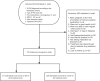
Methods: In this retrospective analysis, we analyzed patients who received oral LE for ovulation induction (OI) at the Hangzhou Women's Hospital from January 2016 to January 2021. In total, 539 PCOS patients with fertility requirements were classified into the D3 group and D5 group according to the different starting times of oral LE, that is, from the 3rd or 5th day of the menstrual cycle or LE is taken orally for 5 days starting on day 3 or 5 of progesterone withdrawal bleeding. Treatment started with one tablet (LE 2.5 mg), continue the regimen from the previous cycle in non-responders and continued until pregnancy or for up to three ovulatory cycles, with visits to determine ovulation and pregnancy, followed by tracking of pregnancies. The primary outcome was to compare ovulation rates, conception rates, live birth rates, pregnancy complications, and pregnancy outcomes at different initiation times.
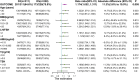
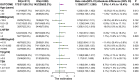
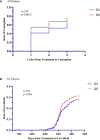
Results: Women who started LE on the 5th day of their menstrual cycle had more cumulative conception rates than those who started LE on the 3rd day(173 of 228[75.9%]vs. 201 of 311[64.6%], P= 0.005; rate ratio for conception, 1.174; 95% confidence interval,1.052 to 1.311) without significant differences in overall live birth rate, though there were 142 of 228[62.3%] in the D5 group versus 172 of 311[55.3%] in the D3 group (P= 0.105). The median (IQR) endometrial thickness was significantly (P = 0.013) greater during the D5 group treatment compared to the D3 group, which may be related to higher conception and clinical pregnancy rates. The median (IQR) maximum follicle diameter was not statistically (P = 0.073) different between the two groups. The cumulative ovulation per cycle rate was higher with D5 than with D3 (287 of 405 treatment cycles [70.9%] vs. 388 of 640 treatment cycles [60.6%], P=0.001). There were no significant between-group differences in pregnancy loss (31 of 173 conceptions in the D5 group [17.9%] and 29 of 201 conceptions in the D3 group [14.4%]) or multiples pregnancy (8.2% and 10.5%, respectively). Rates of other adverse events during pregnancy were similar in the two treatment groups.
Conclusion: As compared with D3 group, D5 group was associated with higher ovulation and conception rates, shorter time-to-pregnancy among infertile women with the PCOS.
Keywords: conception; infertility; letrozole; ovulation induction; polycystic ovarian syndrome; pregnancy.
Copyright © 2022 Shi, Ye, Gao, Chen, Jin and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Letrozole versus clomiphene for infertility in the polycystic ovary syndrome.N Engl J Med. 2014 Jul 10;371(2):119-29. doi: 10.1056/NEJMoa1313517. N Engl J Med. 2014. PMID: 25006718 Free PMC article. Clinical Trial.
-
Double-blind randomized controlled trial of letrozole versus clomiphene citrate in subfertile women with polycystic ovarian syndrome.Hum Reprod. 2017 Aug 1;32(8):1631-1638. doi: 10.1093/humrep/dex227. Hum Reprod. 2017. PMID: 28854590 Free PMC article. Clinical Trial.
-
The Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) trial: rationale and design of a double-blind randomized trial of clomiphene citrate and letrozole for the treatment of infertility in women with polycystic ovary syndrome.Contemp Clin Trials. 2012 May;33(3):470-81. doi: 10.1016/j.cct.2011.12.005. Epub 2012 Jan 13. Contemp Clin Trials. 2012. PMID: 22265923 Free PMC article. Clinical Trial.
-
The use of aromatase inhibitors for ovulation induction.Curr Opin Obstet Gynecol. 2015 Jun;27(3):206-9. doi: 10.1097/GCO.0000000000000163. Curr Opin Obstet Gynecol. 2015. PMID: 25710389 Review.
-
Letrozole versus clomiphene citrate in polycystic ovary syndrome: a meta-analysis of randomized controlled trials.Arch Gynecol Obstet. 2018 May;297(5):1081-1088. doi: 10.1007/s00404-018-4688-6. Epub 2018 Feb 1. Arch Gynecol Obstet. 2018. PMID: 29392438
Cited by
-
Sequential 2.5 mg letrozole/FSH therapy is more effective for promoting pregnancy in infertile women with PCOS: a pragmatic randomized controlled trial.Front Endocrinol (Lausanne). 2024 Jan 15;14:1294339. doi: 10.3389/fendo.2023.1294339. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38283747 Free PMC article. Clinical Trial.
-
Extended versus conventional letrozole regimen in patients with polycystic ovary syndrome undergoing their first ovulation induction cycle: a prospective randomized controlled trial.Hum Reprod Open. 2024 Jul 18;2024(3):hoae046. doi: 10.1093/hropen/hoae046. eCollection 2024. Hum Reprod Open. 2024. PMID: 39105109 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

