The human cytomegalovirus decathlon: Ten critical replication events provide opportunities for restriction
- PMID: 36506089
- PMCID: PMC9732275
- DOI: 10.3389/fcell.2022.1053139
The human cytomegalovirus decathlon: Ten critical replication events provide opportunities for restriction
Abstract
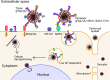
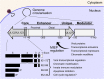
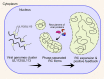
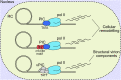
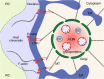
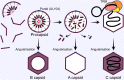
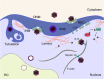
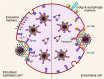
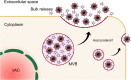
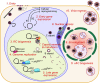
Human cytomegalovirus (HCMV) is a ubiquitous human pathogen that can cause severe disease in immunocompromised individuals, transplant recipients, and to the developing foetus during pregnancy. There is no protective vaccine currently available, and with only a limited number of antiviral drug options, resistant strains are constantly emerging. Successful completion of HCMV replication is an elegant feat from a molecular perspective, with both host and viral processes required at various stages. Remarkably, HCMV and other herpesviruses have protracted replication cycles, large genomes, complex virion structure and complicated nuclear and cytoplasmic replication events. In this review, we outline the 10 essential stages the virus must navigate to successfully complete replication. As each individual event along the replication continuum poses as a potential barrier for restriction, these essential checkpoints represent potential targets for antiviral development.
Keywords: HCMV (human cytomegalovirus); antiviral therapeutic; herpes viral infection; viral replication; virion assembly.
Copyright © 2022 Turner and Mathias.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

