Investigation of the Adsorption of Hydrogen Sulfide on Faujasite Zeolites Focusing on the Influence of Cations
- PMID: 36506121
- PMCID: PMC9730461
- DOI: 10.1021/acsomega.2c04606
Investigation of the Adsorption of Hydrogen Sulfide on Faujasite Zeolites Focusing on the Influence of Cations
Abstract
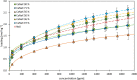
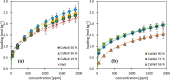
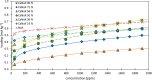
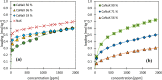
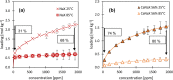
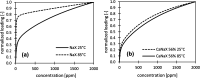
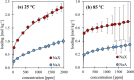
During the conversion of natural gas to liquified natural gas, sulfur components are separated by adsorption on zeolites. New zeolite materials may improve this adsorption process. In this paper, the adsorption of hydrogen sulfide is studied on seven faujasite (FAU) zeolites, which differ only in the number of sodium and calcium cations. From a pure NaX zeolite (13X), which contains only sodium cations, the calcium cation content was gradually increased by ion exchange. In a fixed-bed adsorber, cumulative equilibrium loadings of H2S on these zeolites were determined at concentrations between 50 and 2000 ppm at 25 and 85 °C and 1.3 bar (abs). Adsorption isotherms were analyzed considering the influence of cation positioning in the FAU zeolites. The experimental data indicate a superposition of a chemisorptive and a physisorptive mechanism. At a small number of chemisorptive sites, we conclude a dissociation of hydrogen sulfide and covalent bonding of the proton and the hydrogen sulfide ion to the zeolite lattice. The contribution of chemisorption exhibits a very low temperature dependence, which is typical for nearly irreversible reactions with an equilibrium strongly shifted to one side. With an increase in the proportion of Ca2+ cations, only physisorptive adsorption by electrostatic interaction with the cations in the lattice was observed. A large number of physisorptive sites have a lower energetic value. The share of physisorption strongly depends on temperature, which is characteristic of reversible equilibrium reactions.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- bp. Statistical Review of World Energy, 2021.https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdf....
-
- IEA . Gas, 2020. https://www.iea.org/reports/gas-2020.
-
- bp. Energy Outlook 2020 Edition, 2020.https://www.bp.com/en/global/corporate/energy-economics/energy-outlook/e....
-
- Berg F.; Pasel C.; Eckardt T.; Bathen D. Temperature Swing Adsorption in Natural Gas Processing: A Concise Overview. ChemBioEng Rev 2019, 6, 59–61. 10.1002/cben.201900005. - DOI
-
- Mokhatab S.; Poe W. A., Eds. Handbook of natural gas transmission and processing, 2nd ed.; Gulf Professional Pub, 2012.
LinkOut - more resources
Full Text Sources
Miscellaneous

