Protective effects of mefunidone on ischemia-reperfusion injury/Folic acid-induced acute kidney injury
- PMID: 36506525
- PMCID: PMC9727196
- DOI: 10.3389/fphar.2022.1043945
Protective effects of mefunidone on ischemia-reperfusion injury/Folic acid-induced acute kidney injury
Erratum in
-
Corrigendum: Protective effects of Mefunidone on ischemia-reperfusion injury/folic acid-induced acute kidney injury.Front Pharmacol. 2023 Apr 5;14:1188615. doi: 10.3389/fphar.2023.1188615. eCollection 2023. Front Pharmacol. 2023. PMID: 37089951 Free PMC article.
Abstract
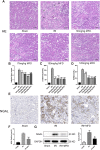
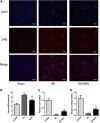
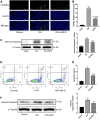
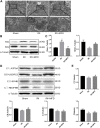
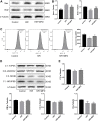
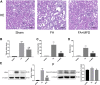
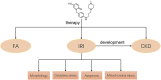
Renal ischemia-reperfusion injury (IRI) is one of the most common causes of acute kidney injury (AKI). It poses a significant threat to public health, and effective therapeutic drugs are lacking. Mefunidone (MFD) is a new pyridinone drug that exerts a significant protective effect on diabetic nephropathy and the unilateral ureteral obstruction (UUO) model in our previous study. However, the effects of mefunidone on ischemia-reperfusion injury-induced acute kidney injury remain unknown. In this study, we investigated the protective effect of mefunidone against ischemia-reperfusion injury-induced acute kidney injury and explored the underlying mechanism. These results revealed that mefunidone exerted a protective effect against ischemia-reperfusion injury-induced acute kidney injury. In an ischemia-reperfusion injury-induced acute kidney injury model, treatment with mefunidone significantly protected the kidney by relieving kidney tubular injury, suppressing oxidative stress, and inhibiting kidney tubular epithelial cell apoptosis. Furthermore, we found that mefunidone reduced mitochondrial damage, regulated mitochondrial-related Bax/bcl2/cleaved-caspase3 apoptotic protein expression, and protected mitochondrial electron transport chain complexes III and V levels both in vivo and in vitro, along with a protective effect on mitochondrial membrane potential in vitro. Given that folic acid (FA)-induced acute kidney injury is a classic model, we used this model to further validate the efficacy of mefunidone in acute kidney injury and obtained the same conclusion. Based on the above results, we conclude that mefunidone has potential protective and therapeutic effects in both ischemia-reperfusion injury- and folic acid-induced acute kidney injury.
Keywords: acute kidney injury; chronic kidney disease; folic acid; mefunidone; renal ischemia-reperfusion injury.
Copyright © 2022 Li, Jiang, Dai, Yu, Lv, Zhang, Liao, Ao, Hu, Meng, Peng, Tao and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Corrigendum: Protective effects of Mefunidone on ischemia-reperfusion injury/folic acid-induced acute kidney injury.Front Pharmacol. 2023 Apr 5;14:1188615. doi: 10.3389/fphar.2023.1188615. eCollection 2023. Front Pharmacol. 2023. PMID: 37089951 Free PMC article.
-
Mefunidone attenuates tubulointerstitial fibrosis in a rat model of unilateral ureteral obstruction.PLoS One. 2015 Jun 4;10(6):e0129283. doi: 10.1371/journal.pone.0129283. eCollection 2015. PLoS One. 2015. PMID: 26042668 Free PMC article.
-
Roxadustat (FG-4592) protects against ischaemia/reperfusion-induced acute kidney injury through inhibiting the mitochondrial damage pathway in mice.Clin Exp Pharmacol Physiol. 2022 Feb;49(2):311-318. doi: 10.1111/1440-1681.13601. Epub 2021 Oct 26. Clin Exp Pharmacol Physiol. 2022. PMID: 34653291
-
Mefunidone ameliorates renal inflammation and tubulointerstitial fibrosis via suppression of IKKβ phosphorylation.Int J Biochem Cell Biol. 2016 Nov;80:109-118. doi: 10.1016/j.biocel.2016.10.005. Epub 2016 Oct 8. Int J Biochem Cell Biol. 2016. PMID: 27725274
-
Extracellular vesicles for ischemia/reperfusion injury-induced acute kidney injury: a systematic review and meta-analysis of data from animal models.Syst Rev. 2022 Sep 8;11(1):197. doi: 10.1186/s13643-022-02003-5. Syst Rev. 2022. PMID: 36076305 Free PMC article.
Cited by
-
Comprehensive overview of the role of mitochondrial dysfunction in the pathogenesis of acute kidney ischemia-reperfusion injury: a narrative review.J Yeungnam Med Sci. 2024 Apr;41(2):61-73. doi: 10.12701/jyms.2023.01347. Epub 2024 Feb 14. J Yeungnam Med Sci. 2024. PMID: 38351610 Free PMC article.
-
Increased Ca2 + transport across the mitochondria-associated membranes by Mfn2 inhibiting endoplasmic reticulum stress in ischemia/reperfusion kidney injury.Sci Rep. 2023 Oct 12;13(1):17257. doi: 10.1038/s41598-023-44538-0. Sci Rep. 2023. PMID: 37828353 Free PMC article.
-
Research on the protection of athletes from injury by flexible conjugated materials in sports events.Front Chem. 2023 Nov 29;11:1313139. doi: 10.3389/fchem.2023.1313139. eCollection 2023. Front Chem. 2023. PMID: 38093817 Free PMC article.
-
Citronellol protects renal function by exerting anti-inflammatory and antiapoptotic effects against acute kidney injury induced by folic acid in mice.Naunyn Schmiedebergs Arch Pharmacol. 2025 May;398(5):5927-5937. doi: 10.1007/s00210-024-03677-5. Epub 2024 Dec 2. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39621091
References
LinkOut - more resources
Full Text Sources
Research Materials

