Appraisal of selected ethnomedicinal plants as alternative therapies against onychomycosis: Evaluation of synergy and time-kill kinetics
- PMID: 36506532
- PMCID: PMC9729263
- DOI: 10.3389/fphar.2022.1067697
Appraisal of selected ethnomedicinal plants as alternative therapies against onychomycosis: Evaluation of synergy and time-kill kinetics
Abstract
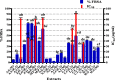
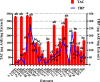
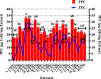
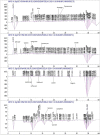
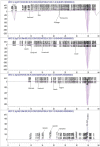
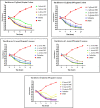
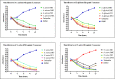
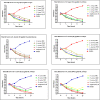
Introduction: This study aims at the biological profiling of Allium sativum, Zingiber officinale, Nigella sativa, Curcuma longa, Mentha piperita, Withania somnifera, Azadirachta indica, and Lawsonia inermis as alternatives against onychomycosis to combat the treatment challenges. Methods: An extract library of aqueous (DW), ethyl acetate (EA), and methanol (M) extracts was subjected to phytochemical and antioxidant colorimetric assays to gauge the ameliorating role of extracts against oxidative stress. RP-HPLC quantified therapeutically significant polyphenols. Antifungal potential (disc diffusion and broth dilution) against filamentous (dermatophytes and non-dermatophytes) and non-filamentous fungi (yeasts; Candida albicans), synergistic interactions (checkerboard method) with terbinafine and amphotericin-B against resistant clinical isolates of dermatophytes (Trichophyton rubrum and Trichophyton tonsurans) and non-dermatophytes (Aspergillus spp., Fusarium dimerum, and Rhizopus arrhizus), time-kill kinetics, and protein estimation (Bradford method) were performed to evaluate the potential of extracts against onychomycosis. Results: The highest total phenolic and flavonoid content along with noteworthy antioxidant capacity, reducing power, and a substantial radical scavenging activity was recorded for the extracts of Z. officinale. Significant polyphenolics quantified by RP-HPLC included rutin (35.71 ± 0.23 µg/mgE), gallic acid (50.17 ± 0.22 µg/mgE), catechin (93.04 ± 0.43 µg/mgE), syringic acid (55.63 ± 0.35 µg/mgE), emodin (246.32 ± 0.44 µg/mgE), luteolin (78.43 ± 0.18 µg/mgE), myricetin (29.44 ± 0.13 µg/mgE), and quercetin (97.45 ± 0.22 µg/mgE). Extracts presented prominent antifungal activity against dermatophytes and non-dermatophytes (MIC-31.25 μg/ml). The checkerboard method showed synergism with 4- and 8-fold reductions in the MICs of A. sativum, Z. officinale, M. piperita, L. inermis, and C. longa extracts and doses of amphotericin-B (Amp-B) and terbinafine (against non-dermatophytes and dermatophytes, respectively). Furthermore, the synergistic therapy showed a time-dependent decrease in fungal growth even after 9 and 12 h of treatment. The inhibition of fungal proteins was also observed to be higher with the treatment of synergistic combinations than with the extracts alone, along with the cell membrane damage caused by terbinafine and amp-B, thus making the resistant fungi incapable of subsisting. Conclusion: The extracts of A. sativum, Z. officinale, M. piperita, L. inermis, and C. longa have proven to be promising alternatives to combat oxidative stress, resistance, and other treatment challenges of onychomycosis.
Keywords: HPLC; antifungal; onychomycosis; resistance; synergistic studies.
Copyright © 2022 Mohsin, Shaukat, Nawaz, Ur-Rehman, Irshad, Majid, Hassan, Bungau and Fatima.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abbas M., Saeed F., Anjum F. M., Afzaal M., Tufail T., Bashir M. S., et al. (2017). Natural polyphenols: An overview. Int. J. Food Prop. 20, 1689–1699. 10.1080/10942912.2016.1220393 - DOI
-
- Abdel-Hameed E.-S. S. (2009). Total phenolic contents and free radical scavenging activity of certain Egyptian Ficus species leaf samples. Food Chem. 114, 1271–1277. 10.1016/j.foodchem.2008.11.005 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

