Postbiotic muramyl dipeptide alleviates colitis via activating autophagy in intestinal epithelial cells
- PMID: 36506547
- PMCID: PMC9727138
- DOI: 10.3389/fphar.2022.1052644
Postbiotic muramyl dipeptide alleviates colitis via activating autophagy in intestinal epithelial cells
Abstract
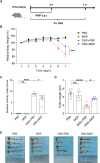
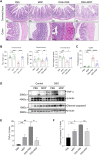
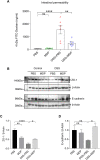
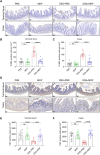
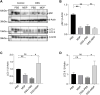
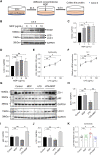
The pathogenesis of IBD is complicated and still unclear. Nucleotide-binding oligomerization domain 2 (NOD2) plays a significant role in regulating gut inflammation under the activation of muramyl dipeptide (MDP), which is used as a postbiotic. The study aimed to investigate the effect of MDP on the intestinal barrier in colitis and the mechanism involved. In this study, C57BL/6 mice were challenged with dextran sodium sulfate (DSS) for establishing a colitis model with the pre-treatment of MDP in vivo. Intestinal permeability was reflected by detecting the serum concentration of 4 kDa Fluorescein Isothiocyanate-Dextran. The expression of inflammation, barrier-related proteins, and autophagy was tested by Western Blotting. Proliferation and apoptosis in intestinal epithelial cells were detected by immunohistochemistry. Caco-2 cells were exposed to lipopolysaccharide for imitating inflammation in vitro. The findings showed that administration of MDP ameliorated losses of body weight loss, gross injury, and histology score of the colon in the DSS-induced colitis mice. MDP significantly ameliorated the condition of gut permeability, and promoted intestinal barrier repair by increasing the expression of Zonula occludens-1 and E-cadherin. Meanwhile, MDP promoted proliferation and reduced apoptosis of intestinal epithelial cells. In the experiment group treated with MDP, LC3 was upregulated, and p62 was downregulated, respectively. These results suggested that MDP stimulation attenuates intestinal inflammation both in vivo and in vitro. Potentially, MDP reduced the intestinal barrier damage by regulating autophagy in intestinal epithelial cells. Future trials investigating the effects of MDP-based postbiotics on IBD may be promising.
Keywords: MDP; NOD2; autophagy; colitis; intestinal barrier.
Copyright © 2022 You, Xiao, Lu, Du, Cai, Cai and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alnabhani Z., Montcuquet N., Biaggini K., Dussaillant M., Roy M., Ogier-Denis E., et al. (2015). Pseudomonas fluorescens alters the intestinal barrier function by modulating IL-1β expression through hematopoietic NOD2 signaling. Inflamm. Bowel Dis. 21 (3), 543–555. 10.1097/mib.0000000000000291 - DOI - PubMed
-
- Arab H. H., Al-Shorbagy M. Y., Saad M. A. (2021). Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem. Biol. Interact. 335, 109368. 10.1016/j.cbi.2021.109368 - DOI - PubMed
LinkOut - more resources
Full Text Sources

