Evaluation and manipulation of tissue and cellular distribution of cardiac progenitor cell-derived extracellular vesicles
- PMID: 36506565
- PMCID: PMC9729535
- DOI: 10.3389/fphar.2022.1052091
Evaluation and manipulation of tissue and cellular distribution of cardiac progenitor cell-derived extracellular vesicles
Abstract
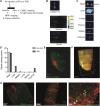
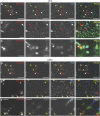
Cardiac progenitor cell-derived extracellular vesicles (CPC-EVs) have been successfully applied via different delivery routes for treating post-myocardial infarction injury in several preclinical models. Hence, understanding the in vivo fate of CPC-EVs after systemic or local, i.e. myocardial, delivery is of utmost importance for the further therapeutic application of CPC-EVs in cardiac repair. Here, we studied the tissue- and cell distribution and retention of CPC-EVs after intramyocardial and intravenous injection in mice by employing different EV labeling and imaging techniques. In contrast to progenitor cells, CPC-EVs demonstrated no immediate flush-out from the heart upon intramyocardial injection and displayed limited distribution to other organs over time, as determined by near-infrared imaging in living animals. By employing CUBIC tissue clearing and light-sheet fluorescent microscopy, we observed CPC-EV migration in the interstitial space of the myocardium shortly after EV injection. Moreover, we demonstrated co-localization with cTnI and CD31-positive cells, suggesting their interaction with various cell types present in the heart. On the contrary, after intravenous injection, most EVs accumulated in the liver. To potentiate such a potential systemic cardiac delivery route, targeting the cardiac endothelium could provide openings for directed CPC-EV therapy. We therefore evaluated whether decorating EVs with targeting peptides (TPs) RGD-4C or CRPPR connected to Lamp2b could enhance EV delivery to endothelial cells. Expression of both TPs enhanced CPC-EV uptake under in vitro continuous flow, but did not affect uptake under static cell culture conditions. Together, these data demonstrate that the route of administration influences CPC-EV biodistribution pattern and suggest that specific TPs could be used to target CPC-EVs to the cardiac endothelium. These insights might lead to a better application of CPC-EV therapeutics in the heart.
Keywords: biodistribution; endothelial cell; exosome; extracellular vesicle; heart; targeting.
Copyright © 2022 Roefs, Heusermann, Brans, Snijders Blok, Lei, Vader and Sluijter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Arslan F., Lai R. C., Smeets M. B., Akeroyd L., Choo A., Aguor E. N. E. E., et al. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 10, 301–312. 10.1016/j.scr.2013.01.002 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

