Erythromycin attenuates oxidative stress-induced cellular senescence via the PI3K-mTOR signaling pathway in chronic obstructive pulmonary disease
- PMID: 36506578
- PMCID: PMC9727195
- DOI: 10.3389/fphar.2022.1043474
Erythromycin attenuates oxidative stress-induced cellular senescence via the PI3K-mTOR signaling pathway in chronic obstructive pulmonary disease
Abstract
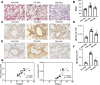
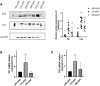
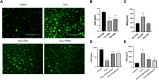
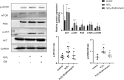
Background and Purpose: Chronic obstructive pulmonary disease (COPD) is proposed to hasten lung aging. Erythromycin protects against oxidative stress and inflammatory responses. However, the potential anti-senescence effect of erythromycin remains disclosed. In the present study, we investigated whether erythromycin influenced oxidative stress-induced cellular senescence and investigated its related mechanisms. Methods: A cigarrete smoke (CS) -induced emphysema mouse model and a H2O2-induced premature senescence model in human bronchial epithelial cell line (BEAS-2B) were established. Senescence-related markers (P53, P21 and SA-β-Gal activity), and levels of oxidative stress biomarkers (MDA, SOD and ROS) were measured. Additionally, cells were pretreated with rapamycin (mTOR inhibitor) or erythromycin, and the expression levels of components of the PI3K-mTOR signaling pathway were measured in BEAS-2B cells. Results: Exposed to H2O2, increased SA-β-gal activity was observed in BEAS-2B cells suggesting premature senescence. Erythromycin inhibited the expression of P53 and P21 in the CS-induced emphysema mouse model. MDA levels significantly increased and SOD levels decreased in the CS-exposed mice and H2O2-induced BEAS-2B cells. Rapamycin and erythromycin significantly suppressed the expression of P53 and P21. Additionally, rapamycin and erythromycin inhibited the PI3K-mTOR signaling pathway. Conclusion: Our findings suggest that erythromycin ameliorates oxidative stress-induced cellular senescence via the PI3K-mTOR signaling pathway. Hence, we establish a theoretical foundation for the clinical application of erythromycin for COPD prevention and treatment.
Keywords: PI3K-mTOR signaling pathway; cellular senescence; chronic obstructive pulmoanry disease; erythromycin; oxidative stress.
Copyright © 2022 Xiaofei, Tingting, Xuan and Zhiyi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Progression of the PI3K/Akt signaling pathway in chronic obstructive pulmonary disease.Front Pharmacol. 2023 Sep 20;14:1238782. doi: 10.3389/fphar.2023.1238782. eCollection 2023. Front Pharmacol. 2023. PMID: 37799975 Free PMC article. Review.
-
Hydrogen sulfide attenuates oxidative stress-induced cellular senescence via the Sirt3/SOD2 signaling pathway in chronic obstructive pulmonary disease.Chin Med J (Engl). 2025 Mar 14. doi: 10.1097/CM9.0000000000003452. Online ahead of print. Chin Med J (Engl). 2025. PMID: 40082252
-
Role of Carbon Monoxide in Oxidative Stress-Induced Senescence in Human Bronchial Epithelium.Oxid Med Cell Longev. 2022 Sep 24;2022:5199572. doi: 10.1155/2022/5199572. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36193088 Free PMC article.
-
Cell-Free Culture Supernatant of Probiotic Lactobacillus fermentum Protects Against H2O2-Induced Premature Senescence by Suppressing ROS-Akt-mTOR Axis in Murine Preadipocytes.Probiotics Antimicrob Proteins. 2020 Jun;12(2):563-576. doi: 10.1007/s12602-019-09576-z. Probiotics Antimicrob Proteins. 2020. PMID: 31332650
-
Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases.Am J Respir Crit Care Med. 2019 Sep 1;200(5):556-564. doi: 10.1164/rccm.201810-1975TR. Am J Respir Crit Care Med. 2019. PMID: 30860857 Review.
Cited by
-
Progression of the PI3K/Akt signaling pathway in chronic obstructive pulmonary disease.Front Pharmacol. 2023 Sep 20;14:1238782. doi: 10.3389/fphar.2023.1238782. eCollection 2023. Front Pharmacol. 2023. PMID: 37799975 Free PMC article. Review.
-
Protective Effect of the Total Alkaloid Extract from Bulbus Fritillariae pallidiflorae in a Mouse Model of Cigarette Smoke-Induced Chronic Obstructive Pulmonary Disease.Int J Chron Obstruct Pulmon Dis. 2024 Jun 10;19:1273-1289. doi: 10.2147/COPD.S459166. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 38881716 Free PMC article.
-
Proteogenomic verifies targets underlying erythromycin alleviate neutrophil extracellular traps-induced inflammation.Respir Res. 2025 Apr 19;26(1):155. doi: 10.1186/s12931-025-03226-5. Respir Res. 2025. PMID: 40253327 Free PMC article.
-
Machine learning models predict the mTOR signal pathway-related signature in the gastric cancer involving 2063 samples of 7 centers.Aging (Albany NY). 2023 Jun 20;15(13):6152-6162. doi: 10.18632/aging.204817. Epub 2023 Jun 20. Aging (Albany NY). 2023. PMID: 37341987 Free PMC article.
-
SenNet recommendations for detecting senescent cells in different tissues.Nat Rev Mol Cell Biol. 2024 Dec;25(12):1001-1023. doi: 10.1038/s41580-024-00738-8. Epub 2024 Jun 3. Nat Rev Mol Cell Biol. 2024. PMID: 38831121 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

