Discovery of highly potent and selective 7-ethyl-10-hydroxycamptothecin-glucose conjugates as potential anti-colorectal cancer agents
- PMID: 36506586
- PMCID: PMC9726873
- DOI: 10.3389/fphar.2022.1014854
Discovery of highly potent and selective 7-ethyl-10-hydroxycamptothecin-glucose conjugates as potential anti-colorectal cancer agents
Abstract
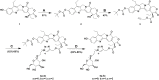
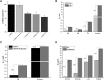
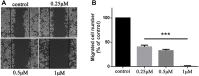
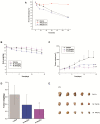
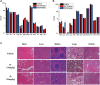
7-Ethyl-10-hydroxycamptothecin (SN38), a highly potent metabolite of irinotecan, has an anticancer efficacy 100-1000 folds more than irinotecan in vitro. However, the clinical application of SN38 has been limited due to the very narrow therapeutic window and poor water solubility. Herein, we report the SN38-glucose conjugates (Glu-SN38) that can target cancer cells due to their selective uptake via glucose transporters, which are overexpressed in most cancers. The in vitro antiproliferative activities against human cancer cell lines and normal cells of Glu-SN38 were investigated. One of the conjugates named 5b showed high potency and selectivity against human colorectal cancer cell line HCT116. Furthermore, 5b remarkably inhibited the growth of HCT116 in vivo. These results suggested that 5b could be a promising drug candidate for treating colorectal cancer.
Keywords: 7-ethyl-10-hydroxycamptothecin; anticancer; colorectal cancer; glucose conjugates; warburg effect.
Copyright © 2022 Yang, Xia, Du, Hu, Gong, Tian, Jiang and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Structural Modification Endows Small-Molecular SN38 Derivatives with Multifaceted Functions.Molecules. 2023 Jun 22;28(13):4931. doi: 10.3390/molecules28134931. Molecules. 2023. PMID: 37446591 Free PMC article. Review.
-
Human serum albumin conjugates of 7-ethyl-10-hydroxycamptothecin (SN38) for cancer treatment.Biomed Res Int. 2014;2014:963507. doi: 10.1155/2014/963507. Epub 2014 May 7. Biomed Res Int. 2014. PMID: 24895635 Free PMC article.
-
Advances in delivery of Irinotecan (CPT-11) active metabolite 7-ethyl-10-hydroxycamptothecin.Int J Pharm. 2019 Sep 10;568:118499. doi: 10.1016/j.ijpharm.2019.118499. Epub 2019 Jul 9. Int J Pharm. 2019. PMID: 31299338 Review.
-
Synthesis and biological evaluation of novel SN38-glucose conjugate for colorectal cancer treatment.Bioorg Med Chem Lett. 2023 Feb 1;81:129128. doi: 10.1016/j.bmcl.2023.129128. Epub 2023 Jan 10. Bioorg Med Chem Lett. 2023. PMID: 36639036
-
Redox responsive 7-ethyl-10-hydroxycamptothecin (SN38) lysophospholipid conjugate: synthesis, assembly and anticancer evaluation.Int J Pharm. 2021 Sep 5;606:120856. doi: 10.1016/j.ijpharm.2021.120856. Epub 2021 Jul 3. Int J Pharm. 2021. PMID: 34229071
Cited by
-
Structural Modification Endows Small-Molecular SN38 Derivatives with Multifaceted Functions.Molecules. 2023 Jun 22;28(13):4931. doi: 10.3390/molecules28134931. Molecules. 2023. PMID: 37446591 Free PMC article. Review.
-
Research Progress of SN38 Drug Delivery System in Cancer Treatment.Int J Nanomedicine. 2024 Jan 26;19:945-964. doi: 10.2147/IJN.S435407. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38293612 Free PMC article. Review.
-
Enhanced Antitumor Efficacy and Reduced Toxicity in Colorectal Cancer Using a Novel Multifunctional Rg3- Targeting Nanosystem Encapsulated with Oxaliplatin and Calcium Peroxide.Int J Nanomedicine. 2025 Jan 24;20:1021-1046. doi: 10.2147/IJN.S502076. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39877588 Free PMC article.
-
Nanomaterials modulate tumor-associated macrophages for the treatment of digestive system tumors.Bioact Mater. 2024 Mar 20;36:376-412. doi: 10.1016/j.bioactmat.2024.03.003. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38544737 Free PMC article. Review.
References
-
- Aiyangar A., Rajput P., Shah B. (2010). Mycophenolate induced diarrhoea. J. Assoc. Physicians India 58, 192–194. - PubMed
LinkOut - more resources
Full Text Sources

