Modified Mayo score versus Mayo score for evaluation of treatment efficacy in patients with ulcerative colitis: data from the tofacitinib OCTAVE program
- PMID: 36506749
- PMCID: PMC9726836
- DOI: 10.1177/17562848221136331
Modified Mayo score versus Mayo score for evaluation of treatment efficacy in patients with ulcerative colitis: data from the tofacitinib OCTAVE program
Abstract
Objectives: The subjectivity of the Physician Global Assessment (PGA) is a limitation of the Mayo score in assessing severity of ulcerative colitis (UC). We compared treatment efficacy using endpoint definitions based on modified Mayo (mMayo) score, versus those based on Mayo score, using data from the tofacitinib OCTAVE program.
Design: This post hoc analysis included data from two 8-week induction studies (OCTAVE Induction 1 and 2) and a 52-week maintenance study (OCTAVE Sustain).
Methods: Remission and clinical response [with nonresponder imputation (NRI)] were assessed using mMayo (without PGA) and Mayo scores, and further stratified by prior tumor necrosis factor inhibitor (TNFi) failure status.
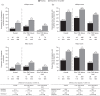
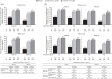
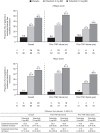
Results: At week 8 of OCTAVE Induction 1 and 2, remission rates with placebo and tofacitinib 10 mg twice daily (BID), respectively, were 7.7% and 24.8% (mMayo) and 6.0% and 17.6% (Mayo). At week 52 of OCTAVE Sustain, remission rates with placebo, tofacitinib 5 and 10 mg BID, respectively, were 12.1%, 35.9%, and 42.1% (mMayo) and 11.1%, 34.3%, and 40.6% (Mayo). A statistically significant (p < 0.05) treatment effect of tofacitinib versus placebo was observed for remission and clinical response at all time points, regardless of scoring definition or prior TNFi failure status.
Conclusions: A significant effect of tofacitinib versus placebo was demonstrated across efficacy endpoints using mMayo score, consistent with previously reported data using Mayo score. Treatment effect sizes were generally similar regardless of scoring definition. This observation may help contextualize tofacitinib therapy outcomes with those of new UC therapies and support the use of Mayo score-based endpoints in UC clinical trials.
Trail registration: ClinicalTrials.gov identifiers: NCT01465763; NCT01458951; NCT01458574.
Keywords: Mayo score; tofacitinib; ulcerative colitis.
© The Author(s), 2022.
Conflict of interest statement
WJS has received grant support, personal fees, and non-financial support from Pfizer Inc during the conduct of the studies. Outside of the submitted work, WJS has received grant support from AbbVie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Eli Lilly, Genentech, Gilead Sciences, GSK, Janssen, Pfizer Inc, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, and Theravance Biopharma; consulting fees from AbbVie, Abivax, Admirx, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Alivio Therapeutics, Allakos, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Bausch Health (Salix), BeiGene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Celgene, Celltrion, Cellularity, Cosmo Pharmaceuticals, Eli Lilly, Equillium, Escalier Biosciences, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic (Vital Therapies), InDex Pharmaceuticals, Intact Therapeutics, Janssen, Kyverna Therapeutics, Landos Biopharma, Oppilan Pharma, Otsuka, Pandion Therapeutics, Pfizer Inc, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Provention Bio, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts, UCB, Vendata Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, and Zealand Pharma; has stock or stock options in Allakos, BeiGene, Gossamer Bio, Oppilan Pharma, Progenity, Prometheus Biosciences, Prometheus Laboratories, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, and Vivreon Biosciences; and is an employee of Shoreline Biosciences. WJS’s spouse has received consulting fees from Iveric Bio, Oppilan Pharma, and Prometheus Laboratories; has stock or stock options in Oppilan Pharma, Progenity, Prometheus Biosciences, Prometheus Laboratories, Ventyx Biosciences, and Vimalan Biosciences; and is an employee of Prometheus Biosciences. BES has received personal fees and non-financial support from Pfizer Inc during the conduct of the studies. Outside of the submitted work, BES has received personal fees from 4D Pharma, AbbVie, Abivax, Allergan, Amgen, Arena Pharmaceuticals, AstraZeneca, Baxalta Bioscience India, Boehringer Ingelheim, Boston Pharmaceuticals, Capella Bioscience, Celgene, Celltrion, Eli Lilly, F. Hoffmann-La Roche, Ferring, Genentech, Gilead Sciences, GSK, Immunic, InDex Pharmaceuticals, Inotrem, Ironwood Pharmaceuticals, Janssen, Johnson & Johnson, Kallyope, Morphic Therapeutic, Oppilan Pharma, OSE Immunotherapeutics, Otsuka, Palatin Technologies, Pfizer Inc, Progenity, Prometheus Biosciences IBD, Prometheus Laboratories, Protagonist Therapeutics, Redhill Biopharma, Rheos Medicines, Salix Pharmaceuticals, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Surrozen, Takeda, TARGET RWE, Theravance Biopharma R&D, USWM Enterprises, Ventyx Biosciences, Viela Bio, and Vivelix Pharmaceuticals; grant support from Arena Pharmaceuticals, Celgene, and Theravance Biopharma R&D; non-financial support from Eli Lilly, Pfizer Inc, and Takeda; and has stock options in Ventyx Biosciences. SV reports grant support from AbbVie, MSD, Pfizer Inc, Galapagos and Takeda; speaker fees from AbbVie, Dr. Falk Pharma, Ferring, Hospira, MSD, Takeda, and Tillotts; and consulting fees from AbbVie, AbolerIS Pharma, Alimentiv, Arena, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Dr Falk Pharma, Eli Lilly, Ferring, Galapagos, Genentech/Roche, Gilead, Hospira, Imidomics, Janssen, Johnson and Johnson, Materia Prima, MiroBio, Morphic, MrMHealth, MSD, Mundipharma, Pfizer Inc, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance Biopharma, Tillots Pharma AG and Zealand Pharma. YL has received consulting fees from AbbVie, Amgen, Janssen, Merck, Pfizer Inc, and Takeda. XG, IM, CS, and WW are employees and shareholders of Pfizer Inc. JP has received personal fees from AbbVie, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Galapagos, Genentech/Roche, GSK, Immunic, Janssen, Origo, Pandion, Pfizer Inc, Progenity, Takeda, Theravance Biopharma, and Wassermann.
Figures

References
-
- D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007; 132: 763–786. - PubMed
-
- U.S. Department of Health and Human Services, Food and Drug Administration and Center for Drug Evaluation and Research (CDER). Ulcerative colitis: clinical trial endpoints. Guidance for industry. http://www.fda.gov/downloads/Drugs/Guidances/UCM515143.pdf (2016, accessed 8 Jul 2021).
-
- Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. - PubMed
-
- Lichtenstein GR, Loftus EV, Jr, Wei SC, et al. Tofacitinib, an oral, small-molecule Janus kinase inhibitor, in the treatment of ulcerative colitis: analysis of an open-label, long-term extension study with up to 5.9 years of treatment [Abstract]. J Crohns Colitis 2020; 14: DOP61.
Associated data
LinkOut - more resources
Full Text Sources
Medical

