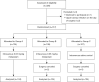
Efficacy of premedication with intravenous midazolam on preoperative anxiety and mask compliance in pediatric patients: a randomized controlled trial
- PMID: 36506775
- PMCID: PMC9732608
- DOI: 10.21037/tp-22-161
Efficacy of premedication with intravenous midazolam on preoperative anxiety and mask compliance in pediatric patients: a randomized controlled trial
Abstract
Background: To alleviate anxiety before surgery is a significant concern for the pediatric anesthesiologist. Midazolam has been generally used as a premedication, and compelling data regarding effective dose to mitigate anxiety is lacking. The current trial addressed the comparable efficacy of intravenous midazolam with different doses regarding the anxiety state, ease of child-parental separation, and mask compliance as premedication in pediatric patients undergoing tonsillectomy.
Methods: Three hundred and twelve children aged 2-8 years were randomly assigned, 104 per group, to receive intravenous 0.03 mg/kg midazolam (group A), 0.05 mg/kg midazolam (group B), or saline control (group C), 40 minutes before surgery. We assessed the anxiety state every 10 min after premedication with modified Yale preoperative anxiety scale (mYPAS), evaluated the emotional state during separation with parental separation anxiety scale (PSAS), and compared their compliance to mask oxygen supply with mask acceptance score (MAS).
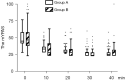
Results: Children premedicated with 0.05 mg/kg midazolam achieved a sedated state more rapidly than those who received 0.03 mg/kg midazolam (5.9±2.3 vs. 7.0±3.9, P=0.02). The proportion of satisfactory parental separation and compliance to mask ventilation was not different between midazolam groups, which was superior to saline control. The children receiving 0.05 mg/kg midazolam stayed longer in postoperative care unit than those receiving 0.03 mg/kg midazolam and saline. The incidence of postoperative adverse events was rare and comparable among groups.
Conclusions: Intravenous administration of a single dose of midazolam 0.05 and 0.03 mg/kg produces similar effects on sedation status, parental separation, and mask induction acceptance, except for rapid-onset and long sedation duration in pediatric patients premedicated with 0.05 mg/kg midazolam.
Trial registration: ClinicalTrials.gov NCT04266340.
Keywords: Anxiety; midazolam; pediatrics; premedication; sedation.
2022 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-161/coif). JJ reports that the conduct of the study and publication of the manuscript was supported by the Shanghai Committee of Science and Technology, with an award (No. 21Y11900400). The other authors have no conflicts of interest to declare.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical