Risk factors for central venous catheter-related thrombosis in hospitalized children: a single-center a retrospective cohort study
- PMID: 36506777
- PMCID: PMC9732607
- DOI: 10.21037/tp-22-529
Risk factors for central venous catheter-related thrombosis in hospitalized children: a single-center a retrospective cohort study
Abstract
Background: This study aimed to explore the risk factors of catheter-related thrombosis (CRT) in children in Southwest China who underwent central venous catheter (CVC) insertion.
Methods: An observational cohort study was conducted at a single tertiary center in southwest China between November 2019 and February 2020. All patients who received a CVC were enrolled and Doppler-ultrasound examination was performed weekly until CVC removal. All patients in this study were hospitalized and were observed and followed up in this hospital. Patient demographics, medication, biochemical indexes, catheter maintenance practice, activities after CVC placement data were analyzed. The Kaplan-Meier method was used to calculate the incidence of CRT, and the Cox regression model was used to analyze the factors influencing CRT.
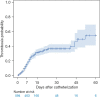
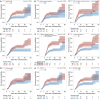
Results: A total of 594 children were included in the study, and the median indwelling time was 10 days, with the shortest being 1 day and the longest 60 days. The overall incidence of CRT was 26.60% (158/594), the 15-day cumulative incidence rate was 30.81%, and the 45-day cumulative incidence rate was 46.27%. After 45 days, the incidence of CRT further increased. Age <12 months [hazard ratio (HR), 1.654; 95% confidence interval (CI): 1.171-2.338], use of 20% mannitol or glycerol fructose (HR, 1.593; 95% CI: 1.058-2.398), CVC placement by a pediatric intensive care unit (PICU) doctor (HR, 1.921; 95% CI: 1.347-2.740), placement length ≥9 cm (HR, 1.633; 95% CI: 1.142-2.336), and D-dimer >1.5 mg/L (HR, 1.451; 95% CI: 1.044-2.015) were risk factors for CRT. Limb exercises (HR, 0.660; 95% CI: 0.469-0.929) after placement was a protective factor for CRT.
Conclusions: The incidence of CRT was higher in children with CVCs, and the key duration of CRT monitoring should be within 15 and 45 days after placement. Patients with age <12 months, using 20% mannitol or glycerol fructose, insertion length ≥9 cm, D-dimer >1.5 mg/L before placement are more likely to happen CVC-CRT than other patient, and it is necessary to be highly vigilant and take preventive measures.
Keywords: Central venous catheter (CVC); catheter-related thrombosis (CRT); children; risk factors.
2022 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-529/coif). The authors have no conflicts of interest to declare.
Figures
References
-
- Östlund Å, Fläring U, Norberg Å, et al. Erratum to 'Incidence of and risk factors for venous thrombosis in children with percutaneous non-tunnelled central venous catheters' (Br J Anaesth 2019; 123: 316-24). Br J Anaesth 2019;123:918. Erratum for: Br J Anaesth 2019;123:316-24. 10.1016/j.bja.2019.09.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
