Effectiveness of BNT162b2 COVID-19 vaccination in prevention of hospitalisations and severe disease in adults with SARS-CoV-2 Delta (B.1.617.2) and Omicron (B.1.1.529) variant between June 2021 and July 2022: A prospective test negative case-control study
- PMID: 36506791
- PMCID: PMC9728025
- DOI: 10.1016/j.lanepe.2022.100552
Effectiveness of BNT162b2 COVID-19 vaccination in prevention of hospitalisations and severe disease in adults with SARS-CoV-2 Delta (B.1.617.2) and Omicron (B.1.1.529) variant between June 2021 and July 2022: A prospective test negative case-control study
Abstract
Background: Whilst other studies have reported the effectiveness of mRNA vaccination against hospitalisation, including emergency department or intensive care admission, few have assessed effectiveness against other more clinically robust indices of COVID-19 severity.
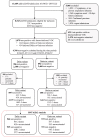
Methods: A prospective single-centre test-negative design case-control study of adults hospitalised with COVID-19 disease or other acute respiratory disease between 1 June 2021 and 20 July 2022. We assessed VE (vaccine effectiveness) against hospitalisation, length of stay [LOS] >3 days, WHO COVID Score >5 and supplementary oxygen FiO2 (fraction inspired oxygen) >28%, conducting regression analyses controlling for age, gender, index of multiple deprivation, Charlson comorbidity index, time, and community infection prevalence.
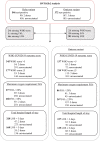
Findings: 935 controls and 546 cases were hospitalised during the Delta period, with 721 controls and 372 cases hospitalised during the Omicron study period. Two-dose BNT162b2 was associated with VE 82.5% [95% confidence interval 76.2%-87.2%] against hospitalisation following Delta infection, 63.3% [26.9-81.8%], 58.5% [24.8-77.3%], and 51.5% [16.7-72.1%] against LOS >3 days, WHO COVID Score >5, and requirement for FiO2 >28% respectively. Three-dose BNT162b2 protection against hospitalisation with Omicron infection was 30.9% [5.9-49.3%], with sensitivity analyses ranging from 28.8-72.6%. Protection against LOS >3 days, WHO COVID Score >5 and requirement for FiO2 >28% was 56.1% [20.6-76.5%], 58.8% [31.2-75.8%], and 41.5% [-0.4-66.3%], respectively. In the UK, BNT162b2 was prioritised for high-risk individuals and those aged >75 years. In the latter group we found a higher estimate of VE against hospitalisation of 47.2% [16.8-66.6%].
Interpretation: BNT162b2 vaccination results in risk reductions for hospitalisation and multiple patient outcomes following Delta and Omicron COVID-19 infection, particularly in older adults. BNT162b2 remains effective against severe SARS-CoV-2 disease.
Funding: AvonCAP is an investigator-led project funded under a collaborative agreement by Pfizer.
Keywords: COVID-19; Respiratory infection; SARS-CoV-2; Vaccination.
© 2022 The Author(s).
Conflict of interest statement
CH is Principal Investigator of the AvonCAP study which is an investigator-led University of Bristol study funded by 10.13039/100004319Pfizer and has previously received support from the NIHR in an Academic Clinical Fellowship. JO and LD are Co-Investigators on the AvonCAP Study. AF is a member of the Joint Committee on Vaccination and Immunization (JCVI) and chair of the World Health Organization European Technical Advisory Group of Experts on Immunization (ETAGE) committee. In addition to receiving funding from 10.13039/100004319Pfizer as Chief Investigator of this study, he leads another project investigating transmission of respiratory bacteria in families jointly funded by 10.13039/100004319Pfizer and the Gates Foundation. The other authors have no relevant conflicts of interest to declare.
Figures
References
-
- Investigation of SARS-CoV-2 variants: technical briefings. https://www.gov.uk/government/publications/investigation-of-sars-cov-2-v... GOV.UK.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

