Dual-Drug Nanosystem: Etoposide Prodrug and Cisplatin Coloaded Nanostructured Lipid Carriers for Lung Cancer Therapy
- PMID: 36506793
- PMCID: PMC9733446
- DOI: 10.2147/DDDT.S386100
Dual-Drug Nanosystem: Etoposide Prodrug and Cisplatin Coloaded Nanostructured Lipid Carriers for Lung Cancer Therapy
Abstract
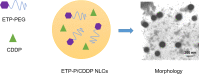
Purpose: Cisplatin (CDDP) and etoposide (Etp) are recommended first-line therapy for lung cancer. Nanostructured lipid carriers (NLCs) are engineered to deliver drugs for lung cancer treatment. In the present study, NLCs were applied to coload an Etp prodrug (EtpP) and CDDP.
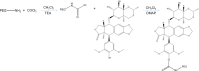
Methods: The Etp prodrug was synthesized by linking the phenolic hydroxyl group of Etp with polyethylene glycol (PEG). EtpP and CDDP coencapsulated NLCs (EtpP-CDDP NLCs) were prepared using film ultrasound. Cytotoxicity of drugs and drug-containing NLCs was assessed by evaluating cell viability using MTT assays. In vivo antitumor efficiency of EtpP-CDDP NLCs was evaluated on lung cancer-bearing xenografts.
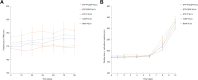
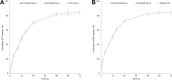
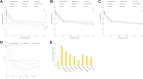
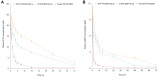
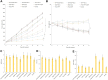
Results: EtpP-CDDP NLCs showed a uniformly spherical morphology with a size of 176.8±4.9 nm and -potential of -31.9±3.2 mV. Cellular uptake efficiency of EtpP-CDDP NLCs was 57.4%±3.9% on A549/DDP cells. EtpP-CDDP NLCs exhibited more sustained plasma retention, the highest drug distribution in tumors, and the highest tumor-inhibition rates in lung tumor-bearing mice.
Conclusion: EtpP-CDDP NLCs improved tumor-cell uptake, cytotoxicity, and tumor-inhibition efficiency, and could be used as a promising drug-delivery system for lung cancer combination therapy.
Keywords: cisplatin; etoposide; lung cancer; nanostructured lipid carriers; prodrug.
© 2022 Du and Yin.
Conflict of interest statement
The authors report no conflicts of interest in relation to this work.
Figures


Similar articles
-
Targeted delivery of etoposide to cancer cells by folate-modified nanostructured lipid drug delivery system.Drug Deliv. 2016 Jun;23(5):1838-45. doi: 10.3109/10717544.2016.1141258. Epub 2016 Feb 16. Drug Deliv. 2016. PMID: 26879035
-
Etoposide-loaded nanostructured lipid carriers for gastric cancer therapy.Drug Deliv. 2016 May;23(4):1379-82. doi: 10.3109/10717544.2015.1048491. Epub 2015 Jul 10. Drug Deliv. 2016. PMID: 26162024
-
Transcription activator, hyaluronic acid and tocopheryl succinate multi-functionalized novel lipid carriers encapsulating etoposide for lymphoma therapy.Biomed Pharmacother. 2017 Jul;91:241-250. doi: 10.1016/j.biopha.2017.04.104. Epub 2017 Apr 28. Biomed Pharmacother. 2017. PMID: 28460227
-
Combination Therapy of Lung Cancer Using Layer-by-Layer Cisplatin Prodrug and Curcumin Co-Encapsulated Nanomedicine.Drug Des Devel Ther. 2020 Jun 9;14:2263-2274. doi: 10.2147/DDDT.S241291. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32606596 Free PMC article.
-
Nanostructured lipid carriers (NLCs): A promising candidate for lung cancer targeting.Int J Pharm. 2024 Apr 25;655:123986. doi: 10.1016/j.ijpharm.2024.123986. Epub 2024 Mar 15. Int J Pharm. 2024. PMID: 38493842 Review.
Cited by
-
Multiple Kinase Small Molecule Inhibitor Tinengotinib (TT-00420) Alone or With Chemotherapy Inhibit the Growth of SCLC.Cancer Sci. 2025 Apr;116(4):951-965. doi: 10.1111/cas.16450. Epub 2025 Jan 16. Cancer Sci. 2025. PMID: 39817471 Free PMC article.
-
Lipid Nanoparticles in Lung Cancer Therapy.Pharmaceutics. 2024 May 10;16(5):644. doi: 10.3390/pharmaceutics16050644. Pharmaceutics. 2024. PMID: 38794306 Free PMC article. Review.
-
Lipid Nanocarrier-Based Drug Delivery Systems: Therapeutic Advances in the Treatment of Lung Cancer.Int J Nanomedicine. 2023 May 18;18:2659-2676. doi: 10.2147/IJN.S406415. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37223276 Free PMC article. Review.
-
Uncovering the Emerging Prospects of Lipid-based Nanoparticulate Vehicles in Lung Cancer Management: A Recent Perspective.Pharm Nanotechnol. 2025;13(1):155-170. doi: 10.2174/0122117385286781240228060152. Pharm Nanotechnol. 2025. PMID: 38468532 Review.
References
-
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. - PubMed
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016, Based on November 2018 SEER Data Submission, Posted to the SEER Web Site, April 2019. Bethesda, MD: National Cancer Institute; 2019.
-
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J; International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

