Advanced nanomaterials for highly efficient CO2 photoreduction and photocatalytic hydrogen evolution
- PMID: 36506822
- PMCID: PMC9733696
- DOI: 10.1080/14686996.2022.2149036
Advanced nanomaterials for highly efficient CO2 photoreduction and photocatalytic hydrogen evolution
Abstract
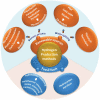
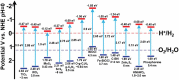
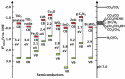
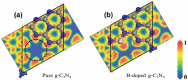
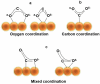
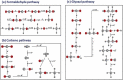
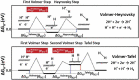
At present, CO2 photoreduction to value-added chemicals/fuels and photocatalytic hydrogen generation by water splitting are the most promising reactions to fix two main issues simultaneously, rising CO2 levels and never-lasting energy demand. CO2, a major contributor to greenhouse gases (GHGs) with about 65% of the total emission, is known to cause adverse effects like global temperature change, ocean acidification, greenhouse effects, etc. The idea of CO2 capture and its conversion to hydrocarbons can control the further rise of CO2 levels and help in producing alternative fuels that have several further applications. On the other hand, hydrogen being a zero-emission fuel is considered as a clean and sustainable form of energy that holds great promise for various industrial applications. The current review focuses on the discussion of the recent progress made in designing efficient photocatalytic materials for CO2 photoreduction and hydrogen evolution reaction (HER). The scope of the current study is limited to the TiO2 and non-TiO2 based advanced nanomaterials (i.e. metal chalcogenides, MOFs, carbon nitrides, single-atom catalysts, and low-dimensional nanomaterials). In detail, the influence of important factors that affect the performance of these photocatalysts towards CO2 photoreduction and HER is reviewed. Special attention is also given in this review to provide a brief account of CO2 adsorption modes on the catalyst surface and its subsequent reduction pathways/product selectivity. Finally, the review is concluded with additional outlooks regarding upcoming research on promising nanomaterials and reactor design strategies for increasing the efficiency of the photoreactions.
Keywords: CO2 reduction; Photocatalysis; TiO2; hydrogen generation; nanomaterials; non-TiO2 materials; water splitting.
© 2022 The Author(s). Published by National Institute for Materials Science in partnership with Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures









Similar articles
-
Designing Nanoengineered Photocatalysts for Hydrogen Generation by Water Splitting and Conversion of Carbon Dioxide to Clean Fuels.Chem Rec. 2022 Sep;22(9):e202200110. doi: 10.1002/tcr.202200110. Epub 2022 Jun 27. Chem Rec. 2022. PMID: 35758532 Review.
-
Photocatalytic nanomaterials and their implications towards biomass conversion for renewable chemical and fuel production.Nanoscale Adv. 2024 Sep 30;6(21):5258-84. doi: 10.1039/d4na00447g. Online ahead of print. Nanoscale Adv. 2024. PMID: 39359352 Free PMC article. Review.
-
Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels.Nanomaterials (Basel). 2020 Feb 17;10(2):337. doi: 10.3390/nano10020337. Nanomaterials (Basel). 2020. PMID: 32079215 Free PMC article. Review.
-
Recent Advances in TiO2-Based Heterojunctions for Photocatalytic CO2 Reduction With Water Oxidation: A Review.Front Chem. 2021 Apr 15;9:637501. doi: 10.3389/fchem.2021.637501. eCollection 2021. Front Chem. 2021. PMID: 33937191 Free PMC article. Review.
-
Insights on Carbon Neutrality by Photocatalytic Conversion of Small Molecules into Value-Added Chemicals or Fuels.Acc Mater Res. 2022 Dec 23;3(12):1206-1219. doi: 10.1021/accountsmr.2c00095. Epub 2022 Nov 4. Acc Mater Res. 2022. PMID: 36583010 Free PMC article.
Cited by
-
Natural Products Derived Porous Carbons for CO2 Capture.Adv Sci (Weinh). 2023 Dec;10(36):e2304289. doi: 10.1002/advs.202304289. Epub 2023 Oct 31. Adv Sci (Weinh). 2023. PMID: 37908147 Free PMC article. Review.
-
Zinc Oxide-Graphitic Carbon Nitride Composites: Synthesis, Properties, and Application Scopes in Environmental Remediations.Small. 2025 Sep;21(36):e09637. doi: 10.1002/smll.202409637. Epub 2025 Jul 25. Small. 2025. PMID: 40709811 Free PMC article. Review.
References
-
- Gupta A, Paul A.. Carbon capture and sequestration potential in India: a comprehensive review. Energy Procedia. 2019;160:848–855.
-
- Kumar P, Laishram D, Sharma RK, et al. Boosting photocatalytic activity using carbon nitride based 2D/2D van der Waals heterojunctions. Chem Mater. 2021;33(23):9012–9092. DOI:10.1021/acs.chemmater.1c03166 - DOI
-
- Draper AM, Weissburg MJ.. Impacts of global warming and elevated CO2 on sensory behavior in predator-prey interactions: a review and synthesis. Front Ecol Evol. 2019;7:72.
-
- Singh G, Kim IY, Lakhi KS, et al. Heteroatom functionalized activated porous biocarbons and their excellent performance for CO2 capture at high pressure. J Mater Chem A. 2017;5(40):21196–21204. DOI:10.1039/C7TA07186H - DOI
-
- Pachauri R, Meyer L. Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. 2014.
Publication types
LinkOut - more resources
Full Text Sources
