Multistudy Research Operations in the ICU: An Interprofessional Pandemic-Informed Approach
- PMID: 36506834
- PMCID: PMC9722625
- DOI: 10.1097/CCE.0000000000000808
Multistudy Research Operations in the ICU: An Interprofessional Pandemic-Informed Approach
Abstract
Proliferation of COVID-19 research underscored the need for improved awareness among investigators, research staff and bedside clinicians of the operational details of clinical studies. The objective was to describe the genesis, goals, participation, procedures, and outcomes of two research operations committees in an academic ICU during the COVID-19 pandemic.
Design: Two-phase, single-center multistudy cohort.
Setting: University-affiliated ICU in Hamilton, ON, Canada.
Patients: Adult patients in the ICU, medical stepdown unit, or COVID-19 ward.
Interventions: None.
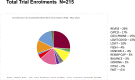
Measurements and main results: An interprofessional COVID Collaborative was convened at the pandemic onset within our department, to proactively coordinate studies, help navigate multiple authentic consent encounters by different research staff, and determine which studies would be suitable for coenrollment. From March 2020 to May 2021, five non-COVID trials continued, two were paused then restarted, and five were launched. Over 15 months, 161 patients were involved in 215 trial enrollments, 110 (51.1%) of which were into a COVID treatment trial. The overall informed consent rate (proportion agreed of those eligible and approached including a priori and deferred consent models) was 83% (215/259). The informed consent rate was lower for COVID-19 trials (110/142, 77.5%) than other trials (105/117, 89.7%; p = 0.01). Patients with COVID-19 were significantly more likely to be coenrolled in two or more studies (29/77, 37.7%) compared with other patients (13/84, 15.5%; p = 0.002). Review items for each new study were collated, refined, and evolved into a modifiable checklist template to set up each study for success. The COVID Collaborative expanded to a more formal Department of Critical Care Research Operations Committee in June 2021, supporting sustainable research operations during and beyond the pandemic.
Conclusions: Structured coordination and increased communication about research operations among diverse research stakeholders cultivated a sense of shared purpose and enhanced the integrity of clinical research operations.
Keywords: clinical research; critical care research; pandemic; randomized trials.
Copyright © 2022 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
Dr Cook holds a Canada Research Chair from the Canadian Institutes for Health Research. Dr. Lewis holds the Constantine Douketis New Researcher Award from the Research Institute of St. Joseph’s Healthcare Hamilton. Dr. Kho holds a Canada Research Chair from the Canadian Institutes for Health Research. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

References
-
- Rome BN, Avorn J: Drug evaluation during the Covid-19 pandemic. N Engl J Med 2020; 382:2282–2284 - PubMed
-
- Duffett M, Cook DJ, Strong G, et al. : The effect of COVID-19 on critical care research: A prospective longitudinal multinational survey. medRxiv Preprint posted online October 23, 2020. doi: 10.1101/2020.10.21.20216945 - DOI
-
- Emanuel EJ, Persad G, Upshur R, et al. : Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med 2020; 382:2049–2055 - PubMed
-
- Peckham S, Hann A: Public Health Ethics and Practice. Bristol, United Kingdom, The Policy Press, 2020
-
- Chong SA, Capps BJ, Subramaniam M, et al. : Clinical research in times of pandemics. Public Health Ethics 2010; 3:35–38
LinkOut - more resources
Full Text Sources
Miscellaneous

