Fluorescence turn-on by photoligation - bright opportunities for soft matter materials
- PMID: 36507164
- PMCID: PMC9682895
- DOI: 10.1039/d2sc05403e
Fluorescence turn-on by photoligation - bright opportunities for soft matter materials
Abstract
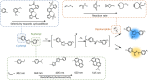
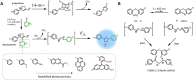
Photochemical ligation has become an indispensable tool for applications that require spatially addressable functionalisation, both in biology and materials science. Interestingly, a number of photochemical ligations result in fluorescent products, enabling a self-reporting function that provides almost instantaneous visual feedback of the reaction's progress and efficiency. Perhaps no other chemical reaction system allows control in space and time to the same extent, while concomitantly providing inherent feedback with regard to reaction success and location. While photoactivable fluorescent properties have been widely used in biology for imaging purposes, the expansion of the array of photochemical reactions has further enabled its utility in soft matter materials. Herein, we concisely summarise the key developments of fluorogenic-forming photoligation systems and their emerging applications in both biology and materials science. We further summarise the current challenges and future opportunities of exploiting fluorescent self-reporting reactions in a wide array of chemical disciplines.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

