Integrated transcriptomic and metabolomic profiling reveals dysregulation of purine metabolism during the acute phase of spinal cord injury in rats
- PMID: 36507345
- PMCID: PMC9727392
- DOI: 10.3389/fnins.2022.1066528
Integrated transcriptomic and metabolomic profiling reveals dysregulation of purine metabolism during the acute phase of spinal cord injury in rats
Abstract
Introduction: Spinal cord injury (SCI) results in drastic dysregulation of microenvironmental metabolism during the acute phase, which greatly affects neural recovery. A better insight into the potential molecular pathways of metabolic dysregulation by multi-omics analysis could help to reveal targets that promote nerve repair and regeneration in the future.
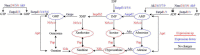
Materials and methods: We established the SCI model and rats were randomly divided into two groups: the acute-phase SCI (ASCI) group (n = 14, 3 days post-SCI) and the sham group with day-matched periods (n = 14, without SCI). In each group, rats were sacrificed at 3 days post-surgery for histology study (n = 3), metabolome sequencing (n = 5), transcriptome sequencing (n = 3), and quantitative real-time polymerase chain reaction (n = 3). The motor function of rats was evaluated by double-blind Basso, Beattie, and Bresnahan (BBB) Locomotor Scores at 0, 1, 2, 3 days post-SCI in an open field area. Then the transcriptomic and metabolomic data were integrated in SCI model of rat to reveal the underlying molecular pathways of microenvironmental metabolic dysregulation.
Results: The histology of the microenvironment was significantly altered in ASCI and the locomotor function was significantly reduced in rats. Metabolomics analysis showed that 360 metabolites were highly altered during the acute phase of SCI, of which 310 were up-regulated and 50 were down-regulated, and bioinformatics analysis revealed that these differential metabolites were mainly enriched in arginine and proline metabolism, D-glutamine and D-glutamate metabolism, purine metabolism, biosynthesis of unsaturated fatty acids. Transcriptomics results showed that 5,963 genes were clearly altered, of which 2,848 genes were up-regulated and 3,115 genes were down-regulated, and these differentially expressed genes were mainly involved in response to stimulus, metabolic process, immune system process. Surprisingly, the Integrative analysis revealed significant dysregulation of purine metabolism at both transcriptome and metabolome levels in the acute phase of SCI, with 48 differential genes and 16 differential metabolites involved. Further analysis indicated that dysregulation of purine metabolism could seriously affect the energy metabolism of the injured microenvironment and increase oxidative stress as well as other responses detrimental to nerve repair and regeneration.
Discussion: On the whole, we have for the first time combined transcriptomics and metabolomics to systematically analyze the potential molecular pathways of metabolic dysregulation in the acute phase of SCI, which will contribute to broaden our understanding of the sophisticated molecular mechanisms of SCI, in parallel with serving as a foundation for future studies of neural repair and regeneration after SCI.
Keywords: integrated analysis; metabolomics; purine metabolism; spinal cord injury; transcriptomics.
Copyright © 2022 Zeng, Li, Jiang, Lan, Chen, Chen, Li, Hui, Zhang, Hu and Xia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources

