Dynamic regulation of phenylpropanoid pathway metabolites in modulating sorghum defense against fall armyworm
- PMID: 36507437
- PMCID: PMC9732255
- DOI: 10.3389/fpls.2022.1019266
Dynamic regulation of phenylpropanoid pathway metabolites in modulating sorghum defense against fall armyworm
Abstract
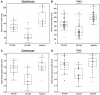
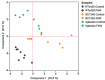
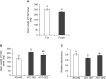
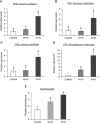
Plants undergo dynamic metabolic changes at the cellular level upon insect infestation to better defend themselves. Phenylpropanoids, a hub of secondary plant metabolites, encompass a wide range of compounds that can contribute to insect resistance. Here, the role of sorghum (Sorghum bicolor) phenylpropanoids in providing defense against the chewing herbivore, fall armyworm (FAW), Spodoptera frugiperda, was explored. We screened a panel of nested association mapping (NAM) founder lines against FAW and identified SC1345 and Ajabsido as most resistant and susceptible lines to FAW, respectively, compared to reference parent, RTx430. Gene expression and metabolomic studies suggested that FAW feeding suppressed the expression level of genes involved in monolignol biosynthetic pathway and their associated phenolic intermediates at 10 days post infestation. Further, SC1345 genotype displayed elevated levels of flavonoid compounds after FAW feeding for 10 days, suggesting a diversion of precursors from lignin biosynthesis to the flavonoid pathway. Additionally, bioassays with sorghum lines having altered levels of flavonoids provided genetic evidence that flavonoids are crucial in providing resistance against FAW. Finally, the application of FAW regurgitant elevated the expression of genes associated with the flavonoid pathway in the FAW-resistant SC1345 genotype. Overall, our study indicates that a dynamic regulation of the phenylpropanoid pathway in sorghum plants imparts resistance against FAW.
Keywords: fall armyworm; flavonoids; plant defense; regurgitant; sorghum.
Copyright © 2022 Grover, Shinde, Puri, Palmer, Sarath, Sattler and Louis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer AB declared a shared affiliation with the authors NP, GS, and SES to the handling editor at the time of review.
Figures




References
-
- Alborn H. T., Turlings T. C. J., Jones T. H., Stenhagen G., Loughrin J. H., Tumlinson, et al. . (1997). An elicitor of plant volatiles from beet armyworm oral secretion. Science 276, 945–949. doi: 10.1126/science.276.5314.945 - DOI
-
- Alon M., Malka O., Eakteiman G., Elbaz M., Zvi M. M. B., Vainstein A., et al. . (2013). Activation of the phenylpropanoid pathway in Nicotiana tabacum improves the performance of the whitefly Bemisia tabaci via reduced jasmonate signaling. PloS One 8, e76619. doi: 10.1371/journal.pone.0076619 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

