Use of platelet-rich plasma for COVID-19-related olfactory loss: a randomized controlled trial
- PMID: 36507615
- PMCID: PMC9877663
- DOI: 10.1002/alr.23116
Use of platelet-rich plasma for COVID-19-related olfactory loss: a randomized controlled trial
Abstract
Introduction: The current study evaluated the use of platelet-rich plasma (PRP), an autologous blood product with supraphysiologic concentrations of growth factors, in the treatment of prolonged coronavirus disease 2019 (COVID-19)-related smell loss.
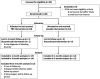
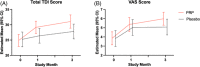
Methods: This multi-institutional, randomized controlled trial recruited patients with COVID-19 who had objectively measured smell loss (University of Pennsylvania Smell Identification Test [UPSIT] ≤ 33) between 6 and 12 months. Patients were randomized to three intranasal injections of either PRP or sterile saline into their olfactory clefts. The primary outcome measure was change in Sniffin' Sticks score (threshold, discrimination, and identification [TDI]) from baseline. The secondary end point measures included responder rate (achievement of a clinically significant improvement, ≥5.5 points TDI), change in individual TDI olfaction scores, and change in subjective olfaction via a visual analog scale.
Results: A total of 35 patients were recruited and 26 completed the study. PRP treatment resulted in a 3.67-point (95% CI: 0.05-7.29, p = 0.047) greater improvement in olfaction compared with the placebo group at 3 months and a higher response rate (57.1% vs 8.3%, odds ratio 12.5 [95% exact bootstrap confidence interval, 2.2-116.7]). There was a greater improvement in smell discrimination following PRP treatment compared with placebo but no difference in smell identification or threshold. There was no difference in subjective scores between PRP and placebo. No adverse effects were reported.
Conclusion: Olfactory function following COVID-19 can improve spontaneously after 6 months and can improve to a greater extent with PRP injection. These data build on the promise of PRP to be a safe potential treatment option for patients with COVID-19-related smell loss, and larger-powered studies will help further assess its efficacy.
Keywords: COVID-19; PRP; anosmia; long COVID; olfaction; persistent olfactory dysfunction; platelet-rich plasma; post COVID syndrome; smell loss; therapeutics.
© 2022 ARS-AAOA, LLC.
Figures
References
-
- Patel ZM, Holbrook EH, Turner JH, et al. International consensus statement on allergy and rhinology: olfaction. Int Forum Allergy Rhinol. 2022;12(4):327‐680. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

