Peptidoglycan Remodeling by an L,D-Transpeptidase, LdtD during Cold Shock in Escherichia coli
- PMID: 36507682
- PMCID: PMC9879098
- DOI: 10.1128/jb.00382-22
Peptidoglycan Remodeling by an L,D-Transpeptidase, LdtD during Cold Shock in Escherichia coli
Abstract
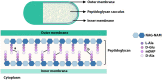
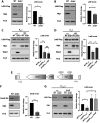
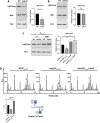
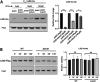
Peptidoglycan (PG) is a unique and essential component of the bacterial cell envelope. It is made up of several linear glycan polymers cross-linked through covalently attached stem peptides making it a fortified mesh-like sacculus around the bacterial cytosolic membrane. In most bacteria, including Escherichia coli, the stem peptide is made up of l-alanine (l-Ala1), d-glutamate (d-Glu2), meso-diaminopimelic acid (mDAP3), d-alanine (d-Ala4), and d-Ala5 with cross-links occurring either between d-ala4 and mDAP3 or between two mDAP3 residues. Of these, the cross-links of the 4-3 (d-Ala4-mDAP3) type are the most predominant and are formed by penicillin-binding D,D-transpeptidases, whereas the formation of less frequent 3-3 linkages (mDAP3-mDAP3) is catalyzed by L,D-transpeptidases. In this study, we found that the frequency of the 3-3 cross-linkages increased upon cold shock in exponentially growing E. coli and that the increase was mediated by an L,D-transpeptidase, LdtD. We found that a cold-inducible RNA helicase DeaD enhanced the cellular LdtD level by facilitating its translation resulting in an increased abundance of 3-3 cross-linkages during cold shock. However, DeaD was also required for optimal expression of LdtD during growth at ambient temperature. Overall, our study finds that E. coli undergoes PG remodeling during cold shock by altering the frequency of 3-3 cross-linkages, implying a role for these modifications in conferring fitness and survival advantage to bacteria growing in diverse environmental conditions. IMPORTANCE Most bacteria are surrounded by a protective exoskeleton called peptidoglycan (PG), an extensively cross-linked mesh-like macromolecule. In bacteria, such as Escherichia coli, the cross-links in the PG are of two types: a major fraction is of 4-3 type whereas a minor fraction is of 3-3 type. Here, we showed that E. coli exposed to cold shock had elevated levels of 3-3 cross-links due to the upregulation of an enzyme, LdtD, that catalyzed their formation. We showed that a cold-inducible RNA helicase DeaD enhanced the cellular LdtD level by facilitating its translation, resulting in increased 3-3 cross-links during cold shock. Our results suggest that PG remodeling contributes to the survival and fitness of bacteria growing in conditions of cold stress.
Keywords: DeaD; L,D-transpeptidases; LdtD; NlpI; cold shock; peptidoglycan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Peptidoglycan hydrolase of an unusual cross-link cleavage specificity contributes to bacterial cell wall synthesis.Proc Natl Acad Sci U S A. 2019 Apr 16;116(16):7825-7830. doi: 10.1073/pnas.1816893116. Epub 2019 Apr 2. Proc Natl Acad Sci U S A. 2019. PMID: 30940749 Free PMC article.
-
Peptidoglycan Remodeling Enables Escherichia coli To Survive Severe Outer Membrane Assembly Defect.mBio. 2019 Feb 5;10(1):e02729-18. doi: 10.1128/mBio.02729-18. mBio. 2019. PMID: 30723128 Free PMC article.
-
Cleavage of Braun's lipoprotein Lpp from the bacterial peptidoglycan by a paralog of l,d-transpeptidases, LdtF.Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2101989118. doi: 10.1073/pnas.2101989118. Proc Natl Acad Sci U S A. 2021. PMID: 33941679 Free PMC article.
-
LD-transpeptidases: the great unknown among the peptidoglycan cross-linkers.FEBS J. 2022 Aug;289(16):4718-4730. doi: 10.1111/febs.16066. Epub 2021 Jun 22. FEBS J. 2022. PMID: 34109739 Review.
-
Breaking down the cell wall: Strategies for antibiotic discovery targeting bacterial transpeptidases.Eur J Med Chem. 2020 May 15;194:112262. doi: 10.1016/j.ejmech.2020.112262. Epub 2020 Mar 23. Eur J Med Chem. 2020. PMID: 32248005 Review.
Cited by
-
Seasonal meropenem resistance in Acinetobacter baumannii and influence of temperature-driven adaptation.BMC Microbiol. 2024 Apr 27;24(1):149. doi: 10.1186/s12866-024-03271-y. BMC Microbiol. 2024. PMID: 38678219 Free PMC article.
-
The distinct transcriptome of virulence-associated phylogenetic group B2 Escherichia coli.Microbiol Spectr. 2023 Sep 19;11(5):e0208523. doi: 10.1128/spectrum.02085-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37724859 Free PMC article.
-
LD-transpeptidase-mediated cell envelope remodeling enables developmental transitions and survival in Coxiella burnetii and Legionella pneumophila.J Bacteriol. 2025 Feb 20;207(2):e0024724. doi: 10.1128/jb.00247-24. Epub 2025 Jan 23. J Bacteriol. 2025. PMID: 39846729 Free PMC article.
References
-
- Weidel W, Pelzer H. 1964. Bagshaped macromolecules-a new outlook on bacterial cell walls, p 193–232. In Advances in Enzymology and Related Areas of Molecular Biology. John Wiley & Sons, Ltd. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

