Estimated public health impact of human rotavirus vaccine (HRV) and pneumococcal polysaccharide protein D-conjugate vaccine (PHiD-CV) on child morbidity and mortality in Gavi-supported countries
- PMID: 36507685
- PMCID: PMC9766466
- DOI: 10.1080/21645515.2022.2135916
Estimated public health impact of human rotavirus vaccine (HRV) and pneumococcal polysaccharide protein D-conjugate vaccine (PHiD-CV) on child morbidity and mortality in Gavi-supported countries
Abstract
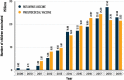
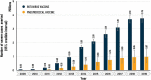
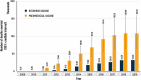
Vaccine impact models against rotavirus disease (RD) and pneumococcal disease (PD) in low- and middle-income countries assume vaccine coverage based on other vaccines. We propose to assess the impact on severe disease cases and deaths avoided based on vaccine doses delivered by one manufacturer to Gavi-supported countries. From the number of human rotavirus vaccine (HRV) and pneumococcal polysaccharide protein D-conjugate vaccine (PHiD-CV) doses delivered, we estimated the averted burden of disease 1) in a specific year and 2) for all children vaccinated during the study period followed-up until 5 years (y) of age. Uncertainty of the estimated impact was assessed in a probabilistic sensitivity analysis using Monte-Carlo simulations to provide 95% confidence intervals. From 2009 to 2019, approximately 143 million children received HRV in 57 Gavi-supported countries, avoiding an estimated 18.7 million severe RD cases and 153,000, deaths. From 2011 to 2019, approximately 146 million children received PHiD-CV in 36 countries, avoiding an estimated 5.0 million severe PD cases and 587,000 deaths. The number of severe cases and deaths averted for all children vaccinated during the study period until 5 years of age were about 23.2 million and 190,000, respectively, for HRV, and 6.6 million and 749,000, respectively, for PHiD-CV. Models based on doses delivered help to assess the impact of vaccination, plan vaccination programs and understand public health benefits. In 2019, HRV and PHiD-CV doses delivered over a 5-y period may have, on average, averted nine severe disease cases every minute and one child death every 4 min.
Keywords: Gavi; Rotavirus; doses delivered; pneumococcal disease; pneumococcal vaccine; rotavirus vaccine.
Plain language summary
What is the context?The WHO added the pneumococcal conjugate vaccine and the rotavirus vaccine in the recommended vaccination schedule of all countries in 2007 and 2009, respectively.Previous studies estimated the public health benefit of these vaccines by approximating the number of children who received them.What is new?We used an alternative approach to estimate the benefit based on actual number of doses of the vaccines, human rotavirus vaccine (HRV; Rotarix) and pneumococcal polysaccharide protein D-conjugate vaccine (PHiD-CV; Synflorix) delivered to each country considered.The study analyzed data from children under 5 years of age in 60 Gavi-supported countries by identifying the number of vaccine doses delivered, estimating the number of children fully covered, applying the country-specific disease epidemiology, estimating the number of severe disease cases and deaths avoided.From 2009 to 2019, approximately 143 million children were vaccinated with HRV avoiding an estimated 18.7 million severe rotavirus disease cases and 153,000 deaths.From 2011 to 2019, about 146 million children were vaccinated with pneumococcal vaccine avoiding an estimated 5.0 million severe pneumococcal disease cases and 587,000 deaths.What is the impact? The benefit of HRV and PHiD-CV in Gavi-supported countries is often estimated based on assumptions of vaccine coverage rates.A modeling approach based on doses delivered by the vaccine manufacturer can provide an additional view on the potential vaccine benefits and improve planning, contribution, and sustainability of the immunization programs at a country level.In 2019, HRV and PHiD-CV together averted nine cases of severe disease each minute and one child death every 4 minutes.
Conflict of interest statement
AM, DVO, LS, MO, PI, PP, and SM are employed by/hold shares in GSK. BS was employed by and held shares in GSK at the moment of the study. All authors declare no other financial or non-financial relationships or activities that could have influenced professional judgment.
Figures



Similar articles
-
Can two different pneumococcal conjugate vaccines be used to complete the infant vaccination series? A randomized trial exploring interchangeability of the 13-valent pneumococcal conjugate vaccine and the pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine.Expert Rev Vaccines. 2020 Nov;19(11):995-1010. doi: 10.1080/14760584.2020.1843431. Expert Rev Vaccines. 2020. PMID: 33297773 Clinical Trial.
-
Immune response to the hepatitis B antigen in the RTS,S/AS01 malaria vaccine, and co-administration with pneumococcal conjugate and rotavirus vaccines in African children: A randomized controlled trial.Hum Vaccin Immunother. 2018 Jun 3;14(6):1489-1500. doi: 10.1080/21645515.2018.1442996. Epub 2018 Apr 13. Hum Vaccin Immunother. 2018. PMID: 29630438 Free PMC article. Clinical Trial.
-
Exploring the evidence behind the comparable impact of the pneumococcal conjugate vaccines PHiD-CV and PCV13 on overall pneumococcal disease.Hum Vaccin Immunother. 2022 Dec 31;18(1):1872341. doi: 10.1080/21645515.2021.1872341. Epub 2021 Feb 19. Hum Vaccin Immunother. 2022. PMID: 33605846 Free PMC article.
-
Ten years of experience with the pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine (Synflorix) in children.Expert Rev Vaccines. 2020 Mar;19(3):247-265. doi: 10.1080/14760584.2020.1738226. Epub 2020 Mar 20. Expert Rev Vaccines. 2020. PMID: 32195602 Review.
-
Pneumococcal polysaccharide protein D-conjugate vaccine (Synflorix; PHiD-CV).Paediatr Drugs. 2009;11(5):349-57. doi: 10.2165/11202760-000000000-00000. Paediatr Drugs. 2009. PMID: 19725600 Review.
Cited by
-
Willingness to Pay for HPV Vaccine among Women Living with HIV in Nigeria.Vaccines (Basel). 2023 May 3;11(5):928. doi: 10.3390/vaccines11050928. Vaccines (Basel). 2023. PMID: 37243032 Free PMC article.
References
-
- World Health Organization . Fact sheet -Immunization coverage. Geneva: Newsroom; 2021. Jul 15 [accessed 2020 Sept 28]. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
-
- World Health Assembly . The expanded programme on immunization: the 1974 resolution by the world health assembly. Assignment Child 1985;69-72:87–11. - PubMed
-
- Gavi the Vaccine Alliance . Eligibility. 2020. Aug 26 [accessed 2021 March 02]. https://www.gavi.org/types-support/sustainability/eligibility.
-
- Gavi The Vaccine Alliance . Facts and figures. 2022. Feb 01 [accessed 2021 Mar 02]. https://www.gavi.org/programmes-impact/our-impact/facts-and-figures.
-
- Gavi The Vaccine Alliance . Evaluation studies. 2021. Dec 21 [accessed 2021 Mar 03]. https://www.gavi.org/programmes-impact/our-impact/evaluation-studies.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
