Serial cross-sectional estimation of vaccine-and infection-induced SARS-CoV-2 seroprevalence in British Columbia, Canada
- PMID: 36507788
- PMCID: PMC9828974
- DOI: 10.1503/cmaj.221335
Serial cross-sectional estimation of vaccine-and infection-induced SARS-CoV-2 seroprevalence in British Columbia, Canada
Abstract
Background: The evolving proportion of the population considered immunologically naive versus primed for more efficient immune memory response to SARS-CoV-2 has implications for risk assessment. We sought to chronicle vaccine- and infection-induced seroprevalence across the first 7 waves of the COVID-19 pandemic in British Columbia, Canada.
Methods: During 8 cross-sectional serosurveys conducted between March 2020 and August 2022, we obtained anonymized residual sera from children and adults who attended an outpatient laboratory network in the Lower Mainland (Greater Vancouver and Fraser Valley). We used at least 3 immunoassays per serosurvey to detect SARS-CoV-2 spike and nucleocapsid antibodies. We assessed any seroprevalence (vaccineor infection-induced, or both), defined by positivity on any 2 assays, and infection-induced seroprevalence, also defined by dual-assay positivity but requiring both antinucleocapsid and antispike detection. We used estimates of infection-induced seroprevalence to explore underascertainment of infections by surveillance case reports.
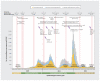
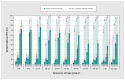
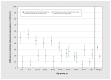
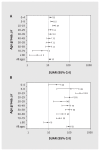
Results: By January 2021, we estimated that any seroprevalence remained less than 5%, increasing with vaccine rollout to 56% by May-June 2021, 83% by September-October 2021 and 95% by March 2022. Infection-induced seroprevalence remained less than 15% through September-October 2021, increasing across Omicron waves to 42% by March 2022 and 61% by July-August 2022. By August 2022, 70%-80% of children younger than 20 years and 60%-70% of adults aged 20-59 years had been infected, but fewer than half of adults aged 60 years and older had been infected. Compared with estimates of infection-induced seroprevalence, surveillance case reports underestimated infections 12-fold between September 2021 and March 2022 and 92-fold between March 2022 and August 2022.
Interpretation: By August 2022, most children and adults younger than 60 years had evidence of both SARS-CoV-2 vaccination and infection. As previous evidence suggests that a history of both exposures may induce stronger, more durable hybrid immunity than either exposure alone, older adults - who have the lowest infection rates but highest risk of severe outcomes - continue to warrant prioritized vaccination.
© 2022 CMA Impact Inc. or its licensors.
Conflict of interest statement
Competing interests: Danuta Skowronski reports grants from the Canadian Institutes of Health Research and the British Columbia Centre for Disease Control Foundation for Public Health, paid to her institution, for other SARS-CoV-2 work. Romina Reyes is chair of the BC Diagnostic Accreditation Program committee. Mel Krajden reports grants paid to his institution from Roche, Hologic and Siemens. No other competing interests were declared.
Figures
References
-
- Skowronski DM, Chambers C, Sabaiduc S, et al. . Pre- and post-pandemic estimates of 2009 pandemic influenza A(H1N1) seroprotection to inform surveillance-based incidence, by age, during the 2013–2014 epidemic in Canada. J Infect Dis 2015;211:109–14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
