Treat-to-target and sequencing therapies in Crohn's disease
- PMID: 36507876
- PMCID: PMC9752313
- DOI: 10.1002/ueg2.12336
Treat-to-target and sequencing therapies in Crohn's disease
Abstract
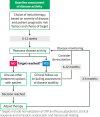
Crohn's disease (CD) is a chronic immune-mediated inflammatory condition which can negatively impact a patient's quality of life. The traditional management strategy for CD has focused on symptomatic control, however, this approach fails to prevent organ damage and to change the progressive course of this disease. Thus, the field has moved towards a treat-to-target strategy that includes identifying individualized objective targets, choosing a therapy based on individual factors that include disease severity and risk, closely monitoring disease activity at predefined time points, and optimizing therapies as needed. Due to the increasing number of therapies approved for CD, this review explores the various factors which should be considered in the sequencing of treatment options together with using the treat-to-target framework to control disease activity early in its course and provide holistic patient care.
Keywords: Crohn's disease; biologic treatments; inflammatory bowel disease; sequencing; treat-to-target.
© 2022 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
NMG has no relevant disclosures. NAC has served as a consultant for Seres Pharmaceuticals and Iterative Scopes. DTR has received grant support from Takeda; and has served as a consultant for Abbvie, Altrubio, Arena Pharmaceuticals, Bristol‐Myers Squibb, Genentech/Roche, Gilead Sciences, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Pfizer, Prometheus Biosciences, Takeda, and Techlab Inc.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

