Iron acquisition strategies in pseudomonads: mechanisms, ecology, and evolution
- PMID: 36508064
- PMCID: PMC10393863
- DOI: 10.1007/s10534-022-00480-8
Iron acquisition strategies in pseudomonads: mechanisms, ecology, and evolution
Abstract
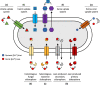
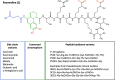
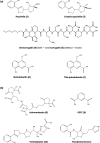
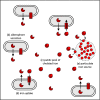
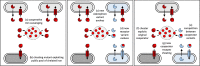
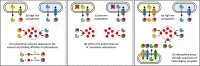
Iron is important for bacterial growth and survival, as it is a common co-factor in essential enzymes. Although iron is very abundant in the earth crust, its bioavailability is low in most habitats because ferric iron is largely insoluble under aerobic conditions and at neutral pH. Consequently, bacteria have evolved a plethora of mechanisms to solubilize and acquire iron from environmental and host stocks. In this review, I focus on Pseudomonas spp. and first present the main iron uptake mechanisms of this taxa, which involve the direct uptake of ferrous iron via importers, the production of iron-chelating siderophores, the exploitation of siderophores produced by other microbial species, and the use of iron-chelating compounds produced by plants and animals. In the second part of this review, I elaborate on how these mechanisms affect interactions between bacteria in microbial communities, and between bacteria and their hosts. This is important because Pseudomonas spp. live in diverse communities and certain iron-uptake strategies might have evolved not only to acquire this essential nutrient, but also to gain relative advantages over competitors in the race for iron. Thus, an integrative understanding of the mechanisms of iron acquisition and the eco-evolutionary dynamics they drive at the community level might prove most useful to understand why Pseudomonas spp., in particular, and many other bacterial species, in general, have evolved such diverse iron uptake repertoires.
Keywords: Citrate; Diversifying selection; Ferrous iron importer; Heme; Siderophore exploitation and competition; Siderophores.
© 2022. The Author(s).
Conflict of interest statement
The author declares that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Figures
References
-
- Albrecht-Gary A-M, Blanc S, Rochel N, Ocaktan AZ, Abdallah MA. Bacterial iron transport: coordination of pyoverdin PaA, a peptidic siderophore of Pseudomonas aeruginosa. Inorg Chem. 1994;33:6391–6402.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

