Association of Direct-Acting Antiviral Therapy With Liver and Nonliver Complications and Long-term Mortality in Patients With Chronic Hepatitis C
- PMID: 36508196
- PMCID: PMC9856614
- DOI: 10.1001/jamainternmed.2022.5699
Association of Direct-Acting Antiviral Therapy With Liver and Nonliver Complications and Long-term Mortality in Patients With Chronic Hepatitis C
Abstract
Importance: Chronic hepatitis C (CHC) and its complications are associated with high rates of morbidity and mortality. However, large-scale data analysis of the long-term liver and nonliver effects of direct-acting antiviral (DAA) treatment has been limited.
Objective: To assess the association of hepatitis C virus elimination through DAA treatment with the risk of liver and nonliver morbidity and mortality during long-term follow-up among a large nationwide cohort of insured patients with CHC in the US.
Design, setting, and participants: This was a retrospective cohort study of 245 596 adult patients with CHC using data from the Optum Clinformatics Data Mart database, 2010 to 2021. Of the total cohort, 40 654 patients had received 1 or more prescriptions for DAA medication (without interferon), and 204 942 patients were untreated.
Exposure: Treatment with a DAA.
Main outcomes and measures: Incidence of hepatocellular carcinoma (HCC), liver decompensation, relevant nonliver events (nonliver cancer, diabetes, chronic kidney disease, cardiovascular disease), and overall mortality.
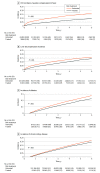
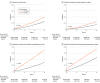
Results: The DAA-treated cohort (vs untreated) were older (mean [SD] age, 59.9 [10.8] vs 58.5 [13.0] years; P < .001); more likely to be male (25 060 [62%] vs 119 727 [58%] men; P < .001) and White (23 937 [59%] vs 115 973 [57%]; P < .001) individuals; and more likely to have diabetes (10 680 [26%] vs 52 091 [25%]; P < .001) or cirrhosis (17 971 [44%] vs 60 094 [29%]; P < .001). Comparing DAA-treated with untreated patients, the incidence (per 1000 person-years) of liver outcomes (eg, decompensation, 28.2 [95% CI, 27.0-29.4] vs 40.8 [95% CI, 40.1-41.5]; P < .001, and HCC in compensated cirrhosis, 20.1 [95% CI, 18.4-21.9] vs 41.8 [95% CI, 40.3-43.3]; P < .001) and nonliver outcomes (eg, diabetes, 30.2 [95% CI, 35.4-37.7] vs 37.2 [95% CI, 36.6-37.9]; P < .001; and chronic kidney disease, 31.1 [95% CI, 29.9-32.2] vs 34.1 [95% CI, 33.5-34.7]; P < .001) were significantly lower in treated patients. The all-cause mortality rates per 1000 person-years were also significantly lower in DAA-treated compared with untreated patients (mortality, 36.5 [95% CI, 35.4-37.7] vs 64.7 [95% CI, 63.9-65.4]; P < .001). In multivariable regression analysis, DAA treatment was independently associated with a significant decrease in the risk of liver (adjusted hazard ratio [aHR] for HCC, 0.73; decompensation, 0.36), nonliver (aHR for diabetes, 0.74; chronic kidney disease, 0.81; cardiovascular disease, 0.90; nonliver cancer, 0.89), and mortality outcomes (aHR, 0.43).
Conclusions and relevance: The findings of this retrospective cohort study indicate that DAA treatment for insured patients with CHC was associated with improved liver- and nonliver outcomes, and ultimately, with long-term overall survival.
Conflict of interest statement
Figures
Comment in
-
Immortal Time and Selection Biases in Study of Direct-Acting Antiviral Treatment and Hepatitis C Outcomes.JAMA Intern Med. 2023 Jun 1;183(6):624. doi: 10.1001/jamainternmed.2023.0554. JAMA Intern Med. 2023. PMID: 37067790 No abstract available.
-
Immortal Time and Selection Biases in Study of Direct-Acting Antiviral Treatment and Hepatitis C Outcomes-Reply.JAMA Intern Med. 2023 Jun 1;183(6):625. doi: 10.1001/jamainternmed.2023.0557. JAMA Intern Med. 2023. PMID: 37067818 No abstract available.
References
-
- Ogawa E, Furusyo N, Kajiwara E, et al. ; Kyushu University Liver Disease Study (KULDS) Group . Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: a prospective, multicenter study. J Hepatol. 2013;58(3):495-501. doi: 10.1016/j.jhep.2012.10.017 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

