Virtual Reality-Augmented Physiotherapy for Chronic Pain in Youth: Protocol for a Randomized Controlled Trial Enhanced With a Single-Case Experimental Design
- PMID: 36508251
- PMCID: PMC9793297
- DOI: 10.2196/40705
Virtual Reality-Augmented Physiotherapy for Chronic Pain in Youth: Protocol for a Randomized Controlled Trial Enhanced With a Single-Case Experimental Design
Abstract
Background: Chronic musculoskeletal (MSK) pain is a prominent health concern, resulting in pain-related disability, loss of functioning, and high health care costs. Physiotherapy rehabilitation is a gold-standard treatment for improving functioning in youth with chronic MSK pain. However, increasing physical activity can feel unattainable for many adolescents because of pain-related fear and movement avoidance. Virtual reality (VR) offers an immersive experience that can interrupt the fear-avoidance cycle and improve engagement in physiotherapy. Despite promising initial findings, data are limited and often lack the rigor required to establish VR as an evidence-based treatment for MSK pain.
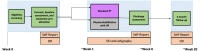
Objective: This trial evaluates physiorehabilitation with VR in adolescents with MSK pain. This protocol outlines the rationale, design, and implementation of a randomized controlled trial enhanced with a single-case experimental design.
Methods: This study is a 2-group randomized controlled trial assessing the use of physiorehabilitation with VR in adolescents with MSK pain. The authors will collaborate with physical therapists to integrate VR into their standard clinical care. For participants enrolled in standard physiotherapy, there will be no VR integrated into their physical therapy program. Primary outcomes include physical function and engagement in VR. Secondary outcomes include pain-related fear and treatment adherence. Moreover, we will obtain clinician perspectives regarding the feasibility of integrating the intervention into the flow of clinical practice.
Results: The pilot study implementing physiorehabilitation with VR demonstrated that high engagement and use of physiorehabilitation with VR were associated with improvements in pain, fear, avoidance, and function. Coupled with qualitative feedback from patients, families, and clinicians, the pilot study results provide support for this trial to evaluate physiorehabilitation with VR for youth with chronic MSK pain. Analysis of results from the main clinical trial will begin as recruitment progresses, and results are expected in early 2024.
Conclusions: Significant breakthroughs for treating MSK pain require mechanistically informed innovative approaches. Physiorehabilitation with VR provides exposure to progressive challenges, real-time feedback, and reinforcement for movement and can include activities that are difficult to achieve in the real world. It has the added benefit of sustaining patient motivation and adherence while enabling clinicians to use objective benchmarks to influence progression. These findings will inform the decision of whether to proceed with a hybrid effectiveness-dissemination trial of physiorehabilitation with VR, serving as the basis for potential large-scale implementation of physiorehabilitation with VR.
Trial registration: ClinicalTrials.gov NCT04636177; https://clinicaltrials.gov/ct2/show/NCT04636177.
International registered report identifier (irrid): DERR1-10.2196/40705.
Keywords: adolescents; chronic pain; mobile phone; physiotherapy; single-case experimental design; virtual reality.
©Laura E Simons, Courtney W Hess, Ellison S Choate, Amanda R Van Orden, Alexandra G Tremblay-McGaw, Maria Menendez, Derek B Boothroyd, Gomathy Parvathinathan, Anya Griffin, Thomas J Caruso, Jennifer Stinson, Amy Weisman, Timothy Liu, Kurt Koeppen. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 12.12.2022.
Conflict of interest statement
Conflicts of Interest: TJC is a recipient of philanthropic donations from Meta, Inc and Magic Leap, Inc.
Figures
Similar articles
-
Implementation of a Virtual Reality Intervention in Outpatient Physiotherapy for Chronic Pain: Protocol for a Pilot Implementation Study.JMIR Res Protoc. 2024 Sep 23;13:e58089. doi: 10.2196/58089. JMIR Res Protoc. 2024. PMID: 39312768 Free PMC article.
-
Examining a Telemedicine-Based Virtual Reality Clinic in Treating Adults With Specific Phobia: Protocol for a Feasibility Randomized Controlled Efficacy Trial.JMIR Res Protoc. 2025 Apr 10;14:e65770. doi: 10.2196/65770. JMIR Res Protoc. 2025. PMID: 40209221 Free PMC article.
-
Treating Lower Phantom Limb Pain in the Postoperative Acute Care Setting Using Virtual Reality: Protocol for a 4-Phase Development and Feasibility Trial.JMIR Res Protoc. 2025 May 23;14:e68008. doi: 10.2196/68008. JMIR Res Protoc. 2025. PMID: 40409745 Free PMC article.
-
Virtual reality simulation training for health professions trainees in gastrointestinal endoscopy.Cochrane Database Syst Rev. 2018 Aug 17;8(8):CD008237. doi: 10.1002/14651858.CD008237.pub3. Cochrane Database Syst Rev. 2018. PMID: 30117156 Free PMC article.
-
The Role of Virtual Reality in Improving Health Outcomes for Community-Dwelling Older Adults: Systematic Review.J Med Internet Res. 2020 Jun 1;22(6):e17331. doi: 10.2196/17331. J Med Internet Res. 2020. PMID: 32478662 Free PMC article.
Cited by
-
Navigating virtual realities: identifying barriers and facilitators to implementing VR-enhanced PT for youth with chronic pain.J Pediatr Psychol. 2025 Jan 1;50(1):76-85. doi: 10.1093/jpepsy/jsae056. J Pediatr Psychol. 2025. PMID: 39110918 Free PMC article.
-
Centering Patient and Clinician Voices in Developing Tools to Address Pain Related School Impairment: A Phase I Study of a Virtual Reality School Simulation for Children and Adolescents with Chronic Pain.Children (Basel). 2023 Oct 1;10(10):1644. doi: 10.3390/children10101644. Children (Basel). 2023. PMID: 37892307 Free PMC article.
-
Acceptance of Virtual Reality in Trainees Using a Technology Acceptance Model: Survey Study.JMIR Med Educ. 2024 Dec 23;10:e60767. doi: 10.2196/60767. JMIR Med Educ. 2024. PMID: 39727193 Free PMC article.
-
Rocket-miR, a translational launchpad for miRNA-based antimicrobial drug development.mSystems. 2023 Dec 21;8(6):e0065323. doi: 10.1128/msystems.00653-23. Epub 2023 Nov 17. mSystems. 2023. PMID: 37975659 Free PMC article.
References
-
- Holley AL, Wilson AC, Palermo TM. Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents: a prospective study. Pain. 2017 May;158(5):794–801. doi: 10.1097/j.pain.0000000000000817. https://europepmc.org/abstract/MED/28151835 - DOI - PMC - PubMed
-
- Groenewald CB, Essner BS, Wright D, Fesinmeyer MD, Palermo TM. The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States. J Pain. 2014 Sep;15(9):925–33. doi: 10.1016/j.jpain.2014.06.002. https://europepmc.org/abstract/MED/24953887 S1526-5900(14)00775-5 - DOI - PMC - PubMed
-
- Murray CB, Groenewald CB, de la Vega R, Palermo TM. Long-term impact of adolescent chronic pain on young adult educational, vocational, and social outcomes. Pain. 2020 Feb;161(2):439–45. doi: 10.1097/j.pain.0000000000001732. https://europepmc.org/abstract/MED/31651579 00006396-202002000-00021 - DOI - PMC - PubMed
-
- Simons LE, Vlaeyen JW, Declercq L, Smith AM, Beebe J, Hogan M, Li E, Kronman CA, Mahmud F, Corey JR, Sieberg CB, Ploski C. Avoid or engage? Outcomes of graded exposure in youth with chronic pain using a sequential replicated single-case randomized design. Pain. 2020 Mar;161(3):520–31. doi: 10.1097/j.pain.0000000000001735. https://europepmc.org/abstract/MED/31693541 00006396-202003000-00009 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

