CureSCi Metadata Catalog-Making sickle cell studies findable
- PMID: 36508412
- PMCID: PMC9744304
- DOI: 10.1371/journal.pone.0256248
CureSCi Metadata Catalog-Making sickle cell studies findable
Abstract
Objectives: To adopt the FAIR principles (Findable, Accessible, Interoperable, Reusable) to enhance data sharing, the Cure Sickle Cell Initiative (CureSCi) MetaData Catalog (MDC) was developed to make Sickle Cell Disease (SCD) study datasets more Findable by curating study metadata and making them available through an open-access web portal.
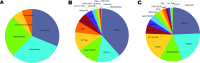
Methods: Study metadata, including study protocol, data collection forms, and data dictionaries, describe information about study patient-level data. We curated key metadata of 16 SCD studies in a three-tiered conceptual framework of category, subcategory, and data element using ontologies and controlled vocabularies to organize the study variables. We developed the CureSCi MDC by indexing study metadata to enable effective browse and search capabilities at three levels: study, Patient-Reported Outcome (PRO) Measures, and data element levels.
Results: The CureSCi MDC offers several browse and search tools to discover studies by study level, PRO Measures, and data elements. The "Browse Studies," "Browse Studies by PRO Measures," and "Browse Studies by Data Elements" tools allow users to identify studies through pre-defined conceptual categories. "Search by Keyword" and "Search Data Element by Concept Category" can be used separately or in combination to provide more granularity to refine the search results. This resource helps investigators find information about specific data elements across studies using public browsing/search tools, before going through data request procedures to access controlled datasets. The MDC makes SCD studies more Findable through browsing/searching study information, PRO Measures, and data elements, aiding in the reuse of existing SCD data.
Copyright: © 2022 Pan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Curesickle.org [Internet]. It’s time to rewrite the story of sickle cell. Available from https://curesickle.org/
-
- Farrell AT, Panepinto J, Desai AA, Kassim AA, Lebensburger J, Walters MC, et al.. End points for sickle cell disease clinical trials: renal and cardiopulmonary, cure, and low-resource settings. Blood Adv. 2019. Dec 10;3(23):4002–20. doi: 10.1182/bloodadvances.2019000883 ; PMCID: PMC6963248. - DOI - PMC - PubMed

