A Drosophila model of HPV16-induced cancer reveals conserved disease mechanism
- PMID: 36508448
- PMCID: PMC9744332
- DOI: 10.1371/journal.pone.0278058
A Drosophila model of HPV16-induced cancer reveals conserved disease mechanism
Abstract
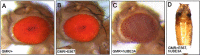
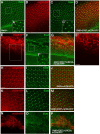
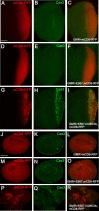
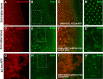
High-risk human papillomaviruses (HR-HPVs) cause almost all cervical cancers and a significant number of vaginal, vulvar, penile, anal, and oropharyngeal cancers. HPV16 and 18 are the most prevalent types among HR-HPVs and together cause more than 70% of all cervical cancers. Low vaccination rate and lack of molecularly-targeted therapeutics for primary therapy have led to a slow reduction in cervical cancer incidence and high mortality rate. Hence, creating new models of HPV-induced cancer that can facilitate understanding of the disease mechanism and identification of key cellular targets of HPV oncogenes are important for development of new interventions. Here in this study, we used the tissue-specific expression technique, Gal4-UAS, to establish the first Drosophila model of HPV16-induced cancer. Using this technique, we expressed HPV16 oncogenes E5, E6, E7 and the human E3 ligase (hUBE3A) specifically in the epithelia of Drosophila eye, which allows simple phenotype scoring without affecting the viability of the organism. We found that, as in human cells, hUBE3A is essential for cellular abnormalities caused by HPV16 oncogenes in flies. Several proteins targeted for degradation by HPV16 oncoproteins in human cells were also reduced in the Drosophila epithelial cells. Cell polarity and adhesion were compromised, resulting in impaired epithelial integrity. Cells did not differentiate to the specific cell types of ommatidia, but instead were transformed into neuron-like cells. These cells extended axon-like structures to connect to each other and exhibited malignant behavior, migrating away to distant sites. Our findings suggest that given the high conservation of genes and signaling pathways between humans and flies, the Drosophila model of HPV16- induced cancer could serve as an excellent model for understanding the disease mechanism and discovery of novel molecularly-targeted therapeutics.
Copyright: © 2022 Hashemi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

