Single-cell analysis of senescent epithelia reveals targetable mechanisms promoting fibrosis
- PMID: 36509292
- PMCID: PMC9746814
- DOI: 10.1172/jci.insight.154124
Single-cell analysis of senescent epithelia reveals targetable mechanisms promoting fibrosis
Abstract
Progressive fibrosis and maladaptive organ repair result in significant morbidity and millions of premature deaths annually. Senescent cells accumulate with aging and after injury and are implicated in organ fibrosis, but the mechanisms by which senescence influences repair are poorly understood. Using 2 murine models of injury and repair, we show that obstructive injury generated senescent epithelia, which persisted after resolution of the original injury, promoted ongoing fibrosis, and impeded adaptive repair. Depletion of senescent cells with ABT-263 reduced fibrosis in reversed ureteric obstruction and after renal ischemia/reperfusion injury. We validated these findings in humans, showing that senescence and fibrosis persisted after relieved renal obstruction. We next characterized senescent epithelia in murine renal injury using single-cell RNA-Seq. We extended our classification to human kidney and liver disease and identified conserved profibrotic proteins, which we validated in vitro and in human disease. We demonstrated that increased levels of protein disulfide isomerase family A member 3 (PDIA3) augmented TGF-β-mediated fibroblast activation. Inhibition of PDIA3 in vivo significantly reduced kidney fibrosis during ongoing renal injury and as such represented a new potential therapeutic pathway. Analysis of the signaling pathways of senescent epithelia connected senescence to organ fibrosis, permitting rational design of antifibrotic therapies.
Keywords: Bioinformatics; Cell Biology; Cellular senescence; Fibrosis; Nephrology.
Figures

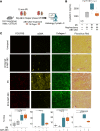








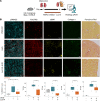
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

