Consensus opinion from an international group of experts on measurable residual disease in hairy cell leukemia
- PMID: 36509740
- PMCID: PMC9744664
- DOI: 10.1038/s41408-022-00760-z
Consensus opinion from an international group of experts on measurable residual disease in hairy cell leukemia
Abstract
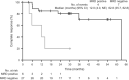
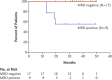
A significant body of literature has been generated related to the detection of measurable residual disease (MRD) at the time of achieving complete remission (CR) in patients with hairy cell leukemia (HCL). However, due to the indolent nature of the disease as well as reports suggesting long-term survival in patients treated with a single course of a nucleoside analog albeit without evidence of cure, the merits of detection of MRD and attempts to eradicate it have been debated. Studies utilizing novel strategies in the relapse setting have demonstrated the utility of achieving CR with undetectable MRD (uMRD) in prolonging the duration of remission. Several assays including immunohistochemical analysis of bone marrow specimens, multi-parameter flow cytometry and molecular assays to detect the mutant BRAF V600E gene or the consensus primer for the immunoglobulin heavy chain gene (IGH) rearrangement have been utilized with few comparative studies. Here we provide a consensus report on the available data, the potential merits of MRD assessment in the front-line and relapse settings and recommendations on future role of MRD assessment in HCL.
© 2022. The Author(s).
Conflict of interest statement
FR: Consultancy and Honoraria: Celgene, BMS, Amgen, Astellas, Xencor, Agios, AstraZeneca, Orsenix, Innate Pharma, Syros, Taiho, Novartis; Research Funding: BMS, Amgen, Xencor, Macrogenics, Orsenix, AbbVie, Taiho, Prelude, Astex. RK: Employment: National Institutes of Health and Regional Cancer Care Associates; Honoraria: PlatformQ, OncLive, Cure; Research Funding: Innate, AstraZeneca, Novartis, Genetech, Pfizer, Teva, Hairy Cell Leukemia Foundation; and Patents: Coinventor for NIH patent for Moxetumomab Pasudotox. EI: Consultant: Innate Pharma. Research funding: Roche. Travel cost: Shire. Holder of a patent on the use of mutant BRAF as HCL biomarker. Our research work in HCL is funded by the Hairy Cell Leukemia Foundation, the Leukemia and Lymphoma Society and the Associazione Italiana Ricerca sul Cancro (AIRC). LA: None. VB: VB serves on the advisory boards of Janssen, Astra Zeneca, AbbVie, and Beigene and has had research funding from CIHR, LLSC, CCMF, Roche, Janssen, and AbbVie. VB also received fees from BIOGEN for patented compounds unrelated to this study. JB: Research funding: AstraZeneca, Oncternal, TG therapeutics, Pharmacyclics/AbbVie; and Advisory Board: AstraZeneca, Pharmacyclics/AbbVie, Beigene, Genentech, Gilead, Innate. SB: Served on Advisory board for Pharmacyclics and Janssen, Beigene and AstraZeneca; Received honorarium from OncLive; and Received travel grant from Arqule. JB: Consulting and Advisory Board: AbbVie, AstraZeneca, INNATE Pharma, KITE Pharma; Research funding: MingSight Pharmaceuticals; and Patents and Intellectual Property: A leukemia diagnostic device (patent pending). AB: None reported. TC: None reported. DC: None reported. CD: Has been an advisor for Medimmune/ Innate Pharma (moxetumomab); Consulting/ Advisory board for AbbVie and Jansen. JD: Has participated in advisory committees in Hungary for Novartis, Bristol Myers Squibb, Amicus, Angelini, Pfizer, Amgen, Roche. SD: None reported. ME: None reported. BF: None reported. FF: Research Support/P.I.: CRUK, Blood Cancer UK; Consultancies: AstraZeneca, AbbVie, Janssen-Cilag, BC-Platform, Beigene; Speakers Bureau: AstraZeneca, AbbVie; and Honoraria: AstraZeneca, AbbVie. DG: None reported. AG: Honoraria: Jansenn and AbbVie. SI: Advisory and speaker fees: Gilead and Takeda; Advisory: Beigene; and Speaker fees: Janssen, Takeda and Gilead. JBJ: None reported. JJ: None reported. GJ: None reported. FL: None reported. GL: None reported. SP: Research funding to the institution from Pharmacyclics, Janssen, AstraZeneca, TG Therapeutics, Merck, AbbVie, and Ascentage Pharma for clinical studies in which Sameer A. Parikh is a principal investigator. Sameer A. Parikh has received honoraria for participation in consulting activities/advisory board meetings for Pharmacyclics, Merck, AstraZeneca, Genentech, GlaxoSmithKline, Adaptive Biotechnologies, and AbbVie (no personal compensation), and from DynaMed, Aptitude Health, Curio Science, and MedEd on the Go (with personal compensation). JP: Research funding from Genentech, Servier, Takeda, Fate Therapeutics and Amgen; Consulting fees from Servier, Amgen, AstraZeneca, Innate Pharma, Novartis, Kite Pharma, Takeda, Intellia, Kura Oncology, Pfizer, BMS, Curocel, Minerva, Autolus; serves on DSMB for Affyimmune and BrightPharma; serves on Scientific Advisory Board for Allogene and Artiva. AP: None reported. GQ: None reported. TR: Honoraria: Sandoz, BeiGene, AstraZeneca; Advisory/Consulting: Jannssen, AstraZeneca, Takeda, BeiGene; and Research Funding: AstraZeneca (ongoing), Acerta (ongoing), Janssen (ongoing). KAR: Research funding: Genentech, AbbVie, Novartis, and Janssen; Consulting: Acerta Pharma, AstraZeneca, Innate Pharma, Genentech, AbbVie, Pharmacyclics, and Beigene; and Travel funding: AstraZeneca. AS: None reported. JS: AbbVie: Advisory board, speakers’ bureau, research funding; Astra Zeneca: Advisory board; Celgene: Advisory board, speakers’ bureau, research funding, expert testimony; Genentech: Advisory board; Gilead: Advisory Board; Janssen: Advisory board, research funding; Mei Pharma: Advisory board; Morphosys, Advisory board; Roche, Advisory board, speakers’ bureau, research funding, expert testimony; Sunesis, Advisory board; Takeda, Advisory board. TT: Grant from Janssen; Advisory Board/Consulting: AbbVie, Janssen, Astra Zeneca, Roche, Novartis, Takeda, and Sanofi. MT: Research funding: AbbVie, Amgen, Biosight, Glycomimetics, Orsenix, Rafael; Advisory boards: Amgen, Daiichi-Sankyo, Innate pharmaceuticals, Jazz, Kahr, Kura, Novartis, Orsenix, Roche, Syros, Ipsen Biopharmaceuticals, and Oncolyze; and Royalties: UpToDate. CT: Honorarium: Beigene, Novartis, LOXO, AbbVie, Janssen; Research funding: Beigene, AbbVie, Janssen. PT: Research Funding: AbbVie, Pharmacyclics, Genentech, Adaptive Biotechnologies; Consultancy: Janssen, Genentech, Adaptive Biotechnologies, AbbVie; Lecture fees: Janssen. XT: Consultant for Innate Pharma, AstraZeneca and Beigene; Advisor for AbbVie. CZ: Research funding to University of Rochester from Acerta/AstraZeneca and TG Therapeutics. TZ: Roche, Innate, Incyte, AbbVie, Gilead, Janssen (honoraria). PLZ: Advisory Board Member: Secura Bio, Celltrion, Gilead, Janssen-Cilag, BMS, Servier, Sandoz, MSD, TG Therapeutics, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, ADC Therapeutics, Incyte, Beigene; Speakers Bureau: Celltrion, Gilead, Janssen-Cilag, BMS, Servier, MSD, TG Therapeutics, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, Incyte, Beigene; Consultant: MSD, Eusapharma, Novartis. BW: None reported. KR: None reported. MG: Consultant: Astra Zeneca, Pharmacyclics, Ascerta, Axio, Inc; Research Funding: Hairy Cell Leukemia Foundation for Patient Data Registry; Travel Expenses: Hairy Cell Leukemia Foundation; Scientific Board: Chair, Hairy Cell Leukemia Foundation Scientific Board (no reimbursement); and Scientific Honorarium: University of Pittsburgh.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

