Ultrafast, autonomous self-healable iontronic skin exhibiting piezo-ionic dynamics
- PMID: 36509757
- PMCID: PMC9744819
- DOI: 10.1038/s41467-022-35434-8
Ultrafast, autonomous self-healable iontronic skin exhibiting piezo-ionic dynamics
Abstract
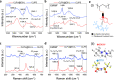
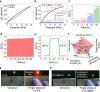
The self-healing properties and ionic sensing capabilities of the human skin offer inspiring groundwork for the designs of stretchable iontronic skins. However, from electronic to ionic mechanosensitive skins, simultaneously achieving autonomously superior self-healing properties, superior elasticity, and effective control of ion dynamics in a homogeneous system is rarely feasible. Here, we report a Cl-functionalized iontronic pressure sensitive material (CLiPS), designed via the introduction of Cl-functionalized groups into a polyurethane matrix, which realizes an ultrafast, autonomous self-healing speed (4.3 µm/min), high self-healing efficiency (91% within 60 min), and mechanosensitive piezo-ionic dynamics. This strategy promotes both an excellent elastic recovery (100%) and effective control of ion dynamics because the Cl groups trap the ions in the system via ion-dipole interactions, resulting in excellent pressure sensitivity (7.36 kPa-1) for tactile sensors. The skin-like sensor responds to pressure variations, demonstrating its potential for touch modulation in future wearable electronics and human-machine interfaces.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Amoli V, et al. Ionic tactile sensors for emerging human-interactive technologies: a review of recent progress. Adv. Funct. Mater. 2020;30:1–32.
-
- Kim JS, Choi H, Hwang HJ, Choi D, Kim DH. All-printed electronic skin based on deformable and ionic mechanotransducer array. Macromol. Biosci. 2020;20:1–7. - PubMed
-
- Jin, M. L. et al. An ultrasensitive, visco-poroelastic artificial mechanotransducer skin inspired by Piezo2 protein in mammalian Merkel cells. Adv. Mater. 29, 1605973 (2017). - PubMed
-
- Chang Y, et al. First decade of interfacial iontronic sensing: from droplet sensors to artificial skins. Adv. Mater. 2021;33:1–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

