Development and validation of a mathematical model of heart rate response to fluid perturbation
- PMID: 36509856
- PMCID: PMC9744837
- DOI: 10.1038/s41598-022-25891-y
Development and validation of a mathematical model of heart rate response to fluid perturbation
Abstract
Physiological closed-loop controlled (PCLC) medical devices monitor and automatically adjust the patient's condition by using physiological variables as feedback, ideally with minimal human intervention to achieve the target levels set by a clinician. PCLC devices present a challenge when it comes to evaluating their performance, where conducting large clinical trials can be expensive. Virtual physiological patients simulated by validated mathematical models can be utilized to obtain pre-clinical evidence of safety and assess the performance of the PCLC medical device during normal and worst-case conditions that are unlikely to happen in a limited clinical trial. A physiological variable that plays a major role during fluid resuscitation is heart rate (HR). For in silico assessment of PCLC medical devices regarding fluid perturbation, there is currently no mathematical model of HR validated in terms of its predictive capability performance. This paper develops and validates a mathematical model of HR response using data collected from sheep subjects undergoing hemorrhage and fluid infusion. The model proved to be accurate in estimating the HR response to fluid perturbation, where averaged between 21 calibration datasets, the fitting performance showed a normalized root mean square error (NRMSE) of [Formula: see text]. The model was also evaluated in terms of model predictive capability performance via a leave-one-out procedure (21 subjects) and an independent validation dataset (6 subjects). Two different virtual cohort generation tools were used in each validation analysis. The generated envelope of virtual subjects robustly met the defined acceptance criteria, in which [Formula: see text] of the testing datasets presented simulated HR patterns that were within a deviation of 50% from the observed data. In addition, out of 16000 and 18522 simulated subjects for the leave-one-out and independent datasets, the model was able to generate at least one virtual subject that was close to the real subject within an error margin of [Formula: see text] and [Formula: see text] NRMSE, respectively. In conclusion, the model can generate valid virtual HR physiological responses to fluid perturbation and be incorporated into future non-clinical simulated testing setups for assessing PCLC devices intended for fluid resuscitation.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures




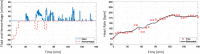

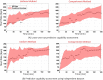
References
-
- Bighamian R, Kim C-S, Reisner AT, Hahn J-O. Closed-loop fluid resuscitation control via blood volume estimation. J. Dyn. Syst. Meas. Control. 2016;138:569. doi: 10.1115/1.4033833. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

