Invasive fungal diseases impact on outcome of childhood ALL - an analysis of the international trial AIEOP-BFM ALL 2009
- PMID: 36509893
- PMCID: PMC9883161
- DOI: 10.1038/s41375-022-01768-x
Invasive fungal diseases impact on outcome of childhood ALL - an analysis of the international trial AIEOP-BFM ALL 2009
Abstract
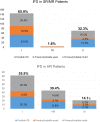
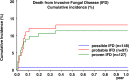
In children with acute lymphoblastic leukemia (ALL), risk groups for invasive fungal disease (IFD) with need for antifungal prophylaxis are not well characterized, and with the advent of new antifungal compounds, current data on outcome are scarce. Prospectively captured serious adverse event reports of children enrolled in the international, multi-center clinical trial AIEOP-BFM ALL2009 were screened for proven/probable IFD, defined according to the updated EORTC/MSG consensus definitions. In a total of 6136 children (median age 5.2 years), 224 proven/probable IFDs (65 yeast and 159 mold) were reported. By logistic regression, the risk for proven/probable IFDs was significantly increased in children ≥12 years and those with a blast count ≥10% in the bone marrow on day 15 (P < 0.0001 each). Proven/probable IFDs had a 6-week and 12-week mortality of 10.7% and 11.2%, respectively. In the multivariate analysis, the hazard ratio for event-free and overall survival was significantly increased for proven/probable IFD, age ≥12 years, and insufficient response to therapy (P < 0.001, each). Our data define older children with ALL and those with insufficient treatment-response at high risk for IFD. As we show that IFD is an independent risk factor for event-free and overall survival, these patients may benefit from targeted antifungal prophylaxis.
© 2022. The Author(s).
Conflict of interest statement
TL has received grants from Gilead Sciences, has served as consultant to Gilead Sciences, Merck/MSD, Pfizer, Astellas, AstraZeneca and Roche, and served at the speaker´s bureau of Gilead Sciences, Merck/MSD, Astellas, Pfizer and GSK. AHG has received grants from Gilead, Merck, Sharp & Dohme and Pfizer and has served as consultant to Amplyx, Astellas, Basilea, F2G, Gilead. Merck, Sharp & Dohme, Pfizer, Scynexis, and Mundipharma. SC served at the speaker´s bureau of Gilead Sciences and Pfizer. AA has received honoraria for lectures, consultancy or advisory board participation from the following companies: Jazz Pharmaceuticals, Amgen, Novartis, MSD, Jazz Pharmaceuticals, Amgen, Novartis, MSD, and Gilead. He has received compensation for travel expenses from Jazz Pharmaceuticals. MS and/or study group have received research support from SHIRE, JazzPharma, Servier, SigmaTau, Amgen, and Novartis. MS has received honoraria from Servier, Novartis, and JazzPharma.
Figures

References
-
- Essig S, Li Q, Chen Y, Hitzler J, Leisenring W, Greenberg M, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2014;15:841–51. doi: 10.1016/S1470-2045(14)70265-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

