SGLT2 inhibitor empagliflozin promotes revascularization in diabetic mouse hindlimb ischemia by inhibiting ferroptosis
- PMID: 36509902
- PMCID: PMC10203292
- DOI: 10.1038/s41401-022-01031-0
SGLT2 inhibitor empagliflozin promotes revascularization in diabetic mouse hindlimb ischemia by inhibiting ferroptosis
Abstract
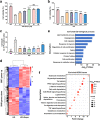
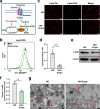
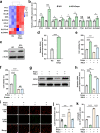
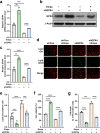
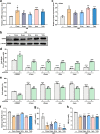
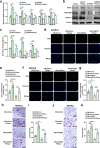
Gliflozins are known as SGLT2 inhibitors, which are used to treat diabetic patients by inhibiting glucose reabsorption in kidney proximal tubules. Recent studies show that gliflozins may exert other effects independent of SGLT2 pathways. In this study we investigated their effects on skeletal muscle cell viability and paracrine function, which were crucial for promoting revascularization in diabetic hindlimb ischemia (HLI). We showed that treatment with empagliflozin (0.1-40 μM) dose-dependently increased high glucose (25 mM)-impaired viability of skeletal muscle C2C12 cells. Canagliflozin, dapagliflozin, ertugliflozin, ipragliflozin and tofogliflozin exerted similar protective effects on skeletal muscle cells cultured under the hyperglycemic condition. Transcriptomic analysis revealed an enrichment of pathways related to ferroptosis in empagliflozin-treated C2C12 cells. We further demonstrated that empagliflozin and other gliflozins (10 μM) restored GPX4 expression in high glucose-treated C2C12 cells, thereby suppressing ferroptosis and promoting cell viability. Empagliflozin (10 μM) also markedly enhanced the proliferation and migration of blood vessel-forming cells by promoting paracrine function of skeletal muscle C2C12 cells. In diabetic HLI mice, injection of empagliflozin into the gastrocnemius muscle of the left hindlimb (10 mg/kg, every 3 days for 21 days) significantly enhanced revascularization and blood perfusion recovery. Collectively, these results reveal a novel effect of empagliflozin, a clinical hypoglycemic gliflozin drug, in inhibiting ferroptosis and enhancing skeletal muscle cell survival and paracrine function under hyperglycemic condition via restoring the expression of GPX4. This study highlights the potential of intramuscular injection of empagliflozin for treating diabetic HLI.
Keywords: diabetic hindlimb ischemia; empagliflozin; ferroptosis; revascularization; therapeutic angiogenesis.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
All authors declare no competing interests. The results of this study have been patented in China (No. ZL202010701077.7).
Figures


References
-
- Isaji M. Sglt2 inhibitors: molecular design and potential differences in effect. Kidney Int Suppl. 2011;120:S14-9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

