Understanding Self-Assembly of Silica-Precipitating Peptides to Control Silica Particle Morphology
- PMID: 36509953
- PMCID: PMC11475327
- DOI: 10.1002/adma.202207586
Understanding Self-Assembly of Silica-Precipitating Peptides to Control Silica Particle Morphology
Abstract
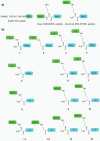
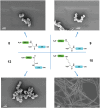
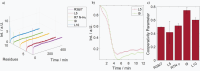
The most advanced materials are those found in nature. These evolutionary optimized substances provide highest efficiencies, e.g., in harvesting solar energy or providing extreme stability, and are intrinsically biocompatible. However, the mimicry of biological materials is limited to a few successful applications since there is still a lack of the tools to recreate natural materials. Herein, such means are provided based on a peptide library derived from the silaffin protein R5 that enables rational biomimetic materials design. It is now evident that biomaterials do not form via mechanisms observed in vitro. Instead, the material's function and morphology are predetermined by precursors that self-assemble in solution, often from a combination of protein and salts. These assemblies act as templates for biomaterials. The RRIL peptides used here are a small part of the silica-precipitation machinery in diatoms. By connecting RRIL motifs via varying central bi- or trifunctional residues, a library of stereoisomers is generated, which allows characterization of different template structures in the presence of phosphate ions by combining residue-resolved real-time NMR spectroscopy and molecular dynamics (MD) simulations. Understanding these templates in atomistic detail, the morphology of silica particles is controlled via manipulation of the template precursors.
Keywords: biomineralization; molecular dynamics; peptide self-assembly; silica particles; silica precipitation.
© 2023 The Authors. Advanced Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Whitesides G. M., Grzybowski B., Science 2002, 295, 2418. - PubMed
-
- Levin A., Hakala T. A., Schnaider L., Bernardes G. J. L., Gazit E., Knowles T. P. J., Nat. Rev. Chem. 2020, 4, 615.
-
- Zelzer M., Ulijn R. V., Chem. Soc. Rev. 2010, 39, 3351. - PubMed
-
- Choi S.‐J., Jeong W.‐J., Kang S.‐K., Lee M., Kim E., Ryu D. Y., Lim Y.‐B., Biomacromolecules 2012, 13, 1991. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

