Intracellular chloride regulation mediates local sleep pressure in the cortex
- PMID: 36510112
- PMCID: PMC7614036
- DOI: 10.1038/s41593-022-01214-2
Intracellular chloride regulation mediates local sleep pressure in the cortex
Abstract
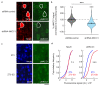
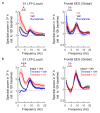
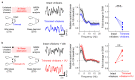
Extended wakefulness is associated with reduced performance and the build-up of sleep pressure. In the cortex, this manifests as changes in network activity. These changes show local variation depending on the waking experience, and their underlying mechanisms represent targets for overcoming the effects of tiredness. Here, we reveal a central role for intracellular chloride regulation, which sets the strength of postsynaptic inhibition via GABAA receptors in cortical pyramidal neurons. Wakefulness results in depolarizing shifts in the equilibrium potential for GABAA receptors, reflecting local activity-dependent processes during waking and involving changes in chloride cotransporter activity. These changes underlie electrophysiological and behavioral markers of local sleep pressure within the cortex, including the levels of slow-wave activity during non-rapid eye movement sleep and low-frequency oscillatory activity and reduced performance levels in the sleep-deprived awake state. These findings identify chloride regulation as a crucial link between sleep-wake history, cortical activity and behavior.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures















References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

