Irisin ameliorates age-associated sarcopenia and metabolic dysfunction
- PMID: 36510115
- PMCID: PMC9891925
- DOI: 10.1002/jcsm.13141
Irisin ameliorates age-associated sarcopenia and metabolic dysfunction
Abstract
Background: Age-associated sarcopenia is characterized of progressed loss of skeletal muscle power, mass, and function, which affects human physical activity and life quality. Besides, accompanied with sarcopenia, aged population also faces a series of metabolic dysfunctions. Irisin, the cleaved form of fibronectin type III domain-containing protein 5 (FNDC5), is a myokine induced by exercise and has been shown to exert multiple beneficial effects on health. The goal of the study is to investigate the alterations of Fndc5/irisin in skeletal muscles during ageing and whether irisin administration could ameliorate age-associated sarcopenia and metabolic dysfunction.
Methods: The mRNA and protein levels of FNDC5/irisin in skeletal muscle and serum from 2- and 24-month-old mice or human subjects were analysed using qRT-PCR and western blot. FNDC5/irisin knockout mice were generated to investigate the consequences of FNDC5/irisin deletion on skeletal muscle mass, as well as morphological and molecular changes in muscle during ageing via histological and molecular analysis. To identify the therapeutic effects of chronic irisin treatment in mice during ageing, in vivo intraperitoneal administration of 2 mg/kg recombinant irisin was performed three times per week in ageing mice (14-month-old) for 4 months or in aged mice (22-month-old) for 1 month to systematically investigate irisin's effects on age-associated sarcopenia and metabolic performances, including grip strength, body weights, body composition, insulin sensitivity, energy expenditure, serum parameters and phenotypical and molecular changes in fat and liver.
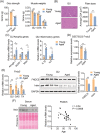
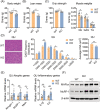
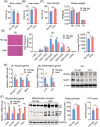
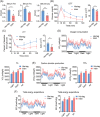
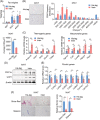
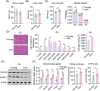
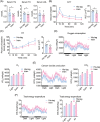
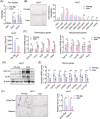
Results: We showed that the expression levels of irisin, as well as its precursor Fndc5, were reduced at mRNA and protein expression levels in muscle during ageing. In addition, via phenotypic analysis of FNDC5/irisin knockout mice, we found that FNDC5/irisin deficiency in aged mice exhibited aggravated muscle atrophy including smaller grip strength (-3.23%, P < 0.05), muscle weights (quadriceps femoris [QU]: -20.05%; gastrocnemius [GAS]: -17.91%; tibialis anterior [TA]: -19.51%, all P < 0.05), fibre size (QU: P < 0.01) and worse molecular phenotypes compared with wild-type mice. We then delivered recombinant irisin protein intraperitoneally into ageing or aged mice and found that it could improve sarcopenia with grip strength (+18.42%, P < 0.01 or +13.88%, P < 0.01), muscle weights (QU: +9.02%, P < 0.01 or +16.39%, P < 0.05), fibre size (QU: both P < 0.05) and molecular phenotypes and alleviated age-associated fat tissues expansion, insulin resistance and hepatic steatosis (all P < 0.05), accompanied with altered gene signatures.
Conclusions: Together, this study revealed the importance of irisin in the maintenance of muscle physiology and systematic energy homeostasis during ageing and suggested a potent therapeutic strategy against age-associated metabolic diseases via irisin administration.
Keywords: FNDC5; ageing; irisin; metabolic dysfunction; sarcopenia.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–2646. - PubMed
-
- Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med 2004;34(0112‐1642 (Print)):809–824. - PubMed
-
- Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014;59:1772–1778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2019YFA0904500/National Key Research and Development Program of China
- 21140904300/Science and Technology Commission of Shanghai Municipality
- SHSMU-ZDCX20212700/Innovative research team of high-level local universities in Shanghai
- 32071148/National Natural Science Foundation of China
- 32271224/National Natural Science Foundation of China
- 32222024/National Natural Science Foundation of China
- 32022034/National Natural Science Foundation of China
- 33906003/Natural Science Foundation of Chongqing, China
- Fundamental Research Funds for the Central Universities
- 011/ECNU public platform for Innovation
- School of Life Sciences, East China Normal University
LinkOut - more resources
Full Text Sources

