Ultra-lung-protective ventilation and biotrauma in severe ARDS patients on veno-venous extracorporeal membrane oxygenation: a randomized controlled study
- PMID: 36510324
- PMCID: PMC9744058
- DOI: 10.1186/s13054-022-04272-x
Ultra-lung-protective ventilation and biotrauma in severe ARDS patients on veno-venous extracorporeal membrane oxygenation: a randomized controlled study
Abstract
Background: Ultra-lung-protective ventilation may be useful during veno-venous extracorporeal membrane oxygenation (vv-ECMO) for severe acute respiratory distress syndrome (ARDS) to minimize ventilator-induced lung injury and to facilitate lung recovery. The objective was to compare pulmonary and systemic biotrauma evaluated by numerous biomarkers of inflammation, epithelial, endothelial injuries, and lung repair according to two ventilator strategies on vv-ECMO.
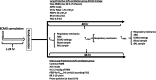
Methods: This is a prospective randomized controlled study. Patients were randomized to receive during 48 h either ultra-lung-protective ventilation combining very low tidal volume (1-2 mL/kg of predicted body weight), low respiratory rate (5-10 cycles per minute), positive expiratory transpulmonary pressure, and 16 h of prone position or lung-protective-ventilation which followed the ECMO arm of the EOLIA trial (control group).
Results: The primary outcome was the alveolar concentrations of interleukin-1-beta, interleukin-6, interleukin-8, surfactant protein D, and blood concentrations of serum advanced glycation end products and angiopoietin-2 48 h after randomization. Enrollment was stopped for futility after the inclusion of 39 patients. Tidal volume, respiratory rate, minute ventilation, plateau pressure, and mechanical power were significantly lower in the ultra-lung-protective group. None of the concentrations of the pre-specified biomarkers differed between the two groups 48 h after randomization. However, a trend to higher 60-day mortality was observed in the ultra-lung-protective group compared to the control group (45 vs 17%, p = 0.06).
Conclusions: Despite a significant reduction in the mechanical power, ultra-lung-protective ventilation during 48 h did not reduce biotrauma in patients with vv-ECMO-supported ARDS. The impact of this ventilation strategy on clinical outcomes warrants further investigation. Trial registration Clinical trial registered with www.
Clinicaltrials: gov ( NCT03918603 ). Registered 17 April 2019.
Keywords: Biotrauma; Severe ARDS; Ultra-lung-protective ventilation; Veno-venous ECMO.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following interests: Christophe Guervilly reported personal consulting fees from Xenios FMC. Matthieu Schmidt received lecture fees from Getinge, Dräger, and Xenios. Françoise Dignat-George and Romaric Lacroix reported grants from Stago and a patent on microvesicle fibrinolytic activity licensed to Stago. Laurent Papazian received consultancy fees from Air Liquide MS, Faron and MSD. All these disclosures are outside the submitted work. The other authors declare no competing interests.
Figures

References
-
- Araos J, Alegria L, Garcia P, Cruces P, Soto D, Erranz B, et al. Near-apneic ventilation decreases lung injury and fibroproliferation in an acute respiratory distress syndrome model with extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2019;199:603–612. doi: 10.1164/rccm.201805-0869OC. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
- 2018-A02297-48/Assistance Publique-Hôpitaux de Marseille
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

