Stereopsis without correspondence
- PMID: 36511401
- PMCID: PMC9745876
- DOI: 10.1098/rstb.2021.0449
Stereopsis without correspondence
Abstract
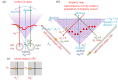
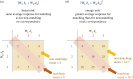
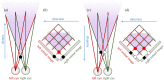
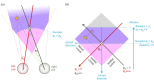
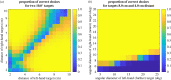
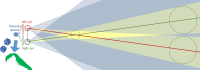
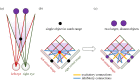
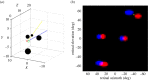
Stereopsis has traditionally been considered a complex visual ability, restricted to large-brained animals. The discovery in the 1980s that insects, too, have stereopsis, therefore, challenged theories of stereopsis. How can such simple brains see in three dimensions? A likely answer is that insect stereopsis has evolved to produce simple behaviour, such as orienting towards the closer of two objects or triggering a strike when prey comes within range. Scientific thinking about stereopsis has been unduly anthropomorphic, for example assuming that stereopsis must require binocular fusion or a solution of the stereo correspondence problem. In fact, useful behaviour can be produced with very basic stereoscopic algorithms which make no attempt to achieve fusion or correspondence, or to produce even a coarse map of depth across the visual field. This may explain why some aspects of insect stereopsis seem poorly designed from an engineering point of view: for example, paying no attention to whether interocular contrast or velocities match. Such algorithms demonstrably work well enough in practice for their species, and may prove useful in particular autonomous applications. This article is part of a discussion meeting issue 'New approaches to 3D vision'.
Keywords: binocular vision; computational neuroscience; evolution; stereopsis.
Figures






References
-
- Smith AM. 1996. Ptolemy's theory of visual perception: an English translation of the ‘Optics’ with introduction and commentary. Trans. Am. Philos. Soc. 86, iii-300. (10.2307/3231951) - DOI
-
- Wade NJ, Ono H. 2012. Early studies of binocular and stereoscopic vision1. Jpn. Psychol. Res. 54, 54-70. (10.1111/j.1468-5884.2011.00505.x) - DOI
-
- Aguilonius F. 1613. Opticorum libri sex. Antwerp, Belgium: Jan Moretus widow & sons.
-
- Boyle R. 1688. A disquisition about the final causes of natural things; wherein it is inquir'd, whether, and (if at all) with what cautions, a naturalist should admit them? To which are subjoyn'd, by way of appendix, some uncommon observations about vitiated sight. London, UK: J. Taylor. See https://wellcomecollection.org/works/bphbffzz.
-
- Wheatstone C. 1838. On some remarkable, and hitherto unobserved, phenomena of binocular vision. Phil. Trans. R. Soc. Lond. B 128, 371-394. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
