Chiral Alcohols from Alkenes and Water: Directed Evolution of a Styrene Hydratase
- PMID: 36511829
- PMCID: PMC10107627
- DOI: 10.1002/anie.202215093
Chiral Alcohols from Alkenes and Water: Directed Evolution of a Styrene Hydratase
Abstract
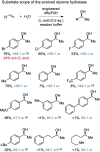
Enantioselective synthesis of chiral alcohols through asymmetric addition of water across an unactivated alkene is a highly sought-after transformation and a big challenge in catalysis. Herein we report the identification and directed evolution of a fatty acid hydratase from Marinitoga hydrogenitolerans for the highly enantioselective hydration of styrenes to yield chiral 1-arylethanols. While directed evolution for styrene hydration was performed in the presence of heptanoic acid to mimic fatty acid binding, the engineered enzyme displayed remarkable asymmetric styrene hydration activity in the absence of the small molecule activator. The evolved styrene hydratase provided access to chiral alcohols from simple alkenes and water with high enantioselectivity (>99 : 1 e.r.) and could be applied on a preparative scale.
Keywords: Alkene Hydration; Biocatalysis; Directed Evolution; Hydratase; Stereoselective Catalysis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

